Glutathione: Lights and Shadows in Cancer Patients
- PMID: 37626722
- PMCID: PMC10452337
- DOI: 10.3390/biomedicines11082226
Glutathione: Lights and Shadows in Cancer Patients
Abstract
In cases of cellular injury, there is an observed increase in the production of reactive oxygen species (ROS). When this production becomes excessive, it can result in various conditions, including cancerogenesis. Glutathione (GSH), the most abundant thiol-containing antioxidant, is fundamental to re-establishing redox homeostasis. In order to evaluate the role of GSH and its antioxi-dant effects in patients affected by cancer, we performed a thorough search on Medline and EMBASE databases for relevant clinical and/or preclinical studies, with particular regard to diet, toxicities, and pharmacological processes. The conjugation of GSH with xenobiotics, including anti-cancer drugs, can result in either of two effects: xenobiotics may lose their harmful effects, or GSH conjugation may enhance their toxicity by inducing bioactivation. While being an interesting weapon against chemotherapy-induced toxicities, GSH may also have a potential protective role for cancer cells. New studies are necessary to better explain the relationship between GSH and cancer. Although self-prescribed glutathione (GSH) implementation is prevalent among cancer patients with the intention of reducing the toxic effects of anticancer treatments and potentially preventing damage to normal tissues, this belief lacks substantial scientific evidence for its efficacy in reducing toxicity, except in the case of cisplatin-related neurotoxicity. Therefore, the use of GSH should only be considered under medical supervision, taking into account the appropriate timing and setting.
Keywords: antioxidants; cancer; chemotherapy; diet; glutathione; nutraceuticals; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

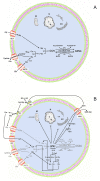

Similar articles
-
Symposium overview: the role of glutathione in neuroprotection and neurotoxicity.Toxicol Sci. 1999 Oct;51(2):161-77. doi: 10.1093/toxsci/51.2.161. Toxicol Sci. 1999. PMID: 10543018 Review.
-
Mitochondrial glutathione and oxidative stress: implications for pulmonary oxygen toxicity in premature infants.Mol Genet Metab. 2000 Sep-Oct;71(1-2):352-8. doi: 10.1006/mgme.2000.3063. Mol Genet Metab. 2000. PMID: 11001827 Review.
-
Comparison of the effects of lipoic acid and glutathione against cisplatin-induced ototoxicity in auditory cells.Int J Pediatr Otorhinolaryngol. 2016 Dec;91:30-36. doi: 10.1016/j.ijporl.2016.10.008. Epub 2016 Oct 8. Int J Pediatr Otorhinolaryngol. 2016. PMID: 27863638
-
The promising anticancer drug 3-bromopyruvate is metabolized through glutathione conjugation which affects chemoresistance and clinical practice: An evidence-based view.Med Hypotheses. 2017 Mar;100:67-77. doi: 10.1016/j.mehy.2017.01.014. Epub 2017 Jan 23. Med Hypotheses. 2017. PMID: 28236852
-
Role of Glutathione in Cancer: From Mechanisms to Therapies.Biomolecules. 2020 Oct 9;10(10):1429. doi: 10.3390/biom10101429. Biomolecules. 2020. PMID: 33050144 Free PMC article. Review.
Cited by
-
Design and In Vitro Activity of Furcellaran/Chitosan Multilayer Microcapsules for the Delivery of Glutathione and Empty Model Multilayer Microcapsules Based on Polysaccharides.Materials (Basel). 2024 Apr 26;17(9):2047. doi: 10.3390/ma17092047. Materials (Basel). 2024. PMID: 38730854 Free PMC article.
-
Identification of disulfidptosis in esophageal squamous cell carcinoma based on single-cell and bulk RNA-seq data to predict prognosis and treatment response.Front Immunol. 2025 Apr 15;16:1567793. doi: 10.3389/fimmu.2025.1567793. eCollection 2025. Front Immunol. 2025. PMID: 40303412 Free PMC article.
-
Influence of Vitamin D and Its Analogues in Type-B Lymphomas.Curr Oncol. 2025 Feb 26;32(3):135. doi: 10.3390/curroncol32030135. Curr Oncol. 2025. PMID: 40136339 Free PMC article. Review.
-
Genome-wide association study and Mendelian randomization analyses reveal insights into bladder cancer etiology.JNCI Cancer Spectr. 2025 Mar 3;9(2):pkaf014. doi: 10.1093/jncics/pkaf014. JNCI Cancer Spectr. 2025. PMID: 39898788 Free PMC article.
-
Disulfidptosis: a novel cell death modality induced by actin cytoskeleton collapse and a promising target for cancer therapeutics.Cell Commun Signal. 2024 Oct 11;22(1):491. doi: 10.1186/s12964-024-01871-9. Cell Commun Signal. 2024. PMID: 39394612 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

