'Spikeopathy': COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA
- PMID: 37626783
- PMCID: PMC10452662
- DOI: 10.3390/biomedicines11082287
'Spikeopathy': COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA
Abstract
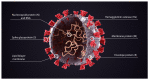
The COVID-19 pandemic caused much illness, many deaths, and profound disruption to society. The production of 'safe and effective' vaccines was a key public health target. Sadly, unprecedented high rates of adverse events have overshadowed the benefits. This two-part narrative review presents evidence for the widespread harms of novel product COVID-19 mRNA and adenovectorDNA vaccines and is novel in attempting to provide a thorough overview of harms arising from the new technology in vaccines that relied on human cells producing a foreign antigen that has evidence of pathogenicity. This first paper explores peer-reviewed data counter to the 'safe and effective' narrative attached to these new technologies. Spike protein pathogenicity, termed 'spikeopathy', whether from the SARS-CoV-2 virus or produced by vaccine gene codes, akin to a 'synthetic virus', is increasingly understood in terms of molecular biology and pathophysiology. Pharmacokinetic transfection through body tissues distant from the injection site by lipid-nanoparticles or viral-vector carriers means that 'spikeopathy' can affect many organs. The inflammatory properties of the nanoparticles used to ferry mRNA; N1-methylpseudouridine employed to prolong synthetic mRNA function; the widespread biodistribution of the mRNA and DNA codes and translated spike proteins, and autoimmunity via human production of foreign proteins, contribute to harmful effects. This paper reviews autoimmune, cardiovascular, neurological, potential oncological effects, and autopsy evidence for spikeopathy. With many gene-based therapeutic technologies planned, a re-evaluation is necessary and timely.
Keywords: COVID-19; autopsy; biodistribution; inflammation; lipid-nanoparticles; mRNA vaccines; pathology; pharmacovigilance; spike protein; transfection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Therapeutic Goods Administration (TGA) FOI Reply 2389-6, p.45 Nonclinical Evaluation Report: BNT162b2 [mRNA] COVID-19 Vaccine (COMIRNATYTM). Submission No: PM-2020-05461-1-2. Sponsor: Pfizer Australia Pty Ltd. Australian Government Department of Health and Aged Care: 2021; FOI reply 2389-6. [(accessed on 7 April 2023)]; Available online: https://www.tga.gov.au/sites/default/files/foi-2389-06.pdf.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

