Thrombosis and Hyperinflammation in COVID-19 Acute Phase Are Related to Anti-Phosphatidylserine and Anti-Phosphatidylinositol Antibody Positivity
- PMID: 37626797
- PMCID: PMC10452204
- DOI: 10.3390/biomedicines11082301
Thrombosis and Hyperinflammation in COVID-19 Acute Phase Are Related to Anti-Phosphatidylserine and Anti-Phosphatidylinositol Antibody Positivity
Abstract
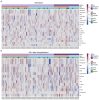
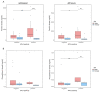
Antiphospholipid antibodies (APLA) are strongly associated with thrombosis seen in patients with antiphospholipid syndrome. In COVID-19, thrombosis has been observed as one of the main comorbidities. In patients hospitalised for COVID-19, we want to check whether APLA positivity is associated with COVID-19-related thrombosis, inflammation, severity of disease, or long COVID-19. We enrolled 92 hospitalised patients with COVID-19 between March and April 2020 who were tested for 18 different APLAs (IgG and IgM) with a single line-immunoassay test. A total of 30 healthy blood donors were used to set the cut-off for each APLA positivity. Of the 92 COVID-19 inpatients, 30 (32.61%; 95% CI [23.41-43.29]) tested positive for APLA, of whom 10 (33.3%; 95% CI [17.94-52.86]) had more than one APLA positivity. Anti-phosphatidylserine IgM positivity was described in 5.4% of inpatients (n = 5) and was associated with the occurrence of COVID-19-related thrombosis (p = 0.046). Anti-cardiolipin IgM positivity was the most prevalent among the inpatients (n = 12, 13.0%) and was associated with a recorded thrombosis in their clinical history (p = 0.044); however, its positivity was not associated with the occurrence of thrombosis during their hospitalisation for COVID-19. Anti-phosphatidylinositol IgM positivity, with a prevalence of 5.4% (n = 5), was associated with higher levels of interleukin (IL)-6 (p = 0.007) and ferritin (p = 0.034). Neither of these APLA positivities was a risk factor for COVID-19 severity or a predictive marker for long COVID-19. In conclusion, almost a third of COVID-19 inpatients tested positive for at least one APLA. Anti-phosphatidylserine positivity in IgM class was associated with thrombosis, and anti-phosphatidylinositol positivity in IgM class was associated with inflammation, as noticed by elevated levels of IL-6. Thus, testing for non-criteria APLA to assess the risk of clinical complications in hospitalised COVID-19 patients might be beneficial. However, they were not related to disease severity or long COVID-19.
Keywords: COVID-19; anti-phosphatidylinositol antibody; anti-phosphatidylserine antibody; antiphospholipid antibodies; disease severity; long COVID-19; thrombosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Antiphospholipid antibodies in children with systemic lupus erythematosus: a long-term clinical and laboratory follow-up status study from northwest India.Rheumatol Int. 2014 May;34(5):669-73. doi: 10.1007/s00296-013-2736-x. Epub 2013 Apr 7. Rheumatol Int. 2014. PMID: 23563494
-
Antiphospholipid Antibodies and Vascular Thrombosis in Patients with Severe Forms of COVID-19.Biomedicines. 2023 Nov 22;11(12):3117. doi: 10.3390/biomedicines11123117. Biomedicines. 2023. PMID: 38137338 Free PMC article.
-
Antiphospholipid antibodies and vitamin D deficiency in COVID-19 infection with and without venous or arterial thrombosis: A pilot case-control study.PLoS One. 2022 Jul 14;17(7):e0269466. doi: 10.1371/journal.pone.0269466. eCollection 2022. PLoS One. 2022. PMID: 35834511 Free PMC article.
-
[The antiphospholipid syndrome--yesterday, today, tomorrow].Vojnosanit Pregl. 1998 Mar-Apr;55(2 Suppl):5-12. Vojnosanit Pregl. 1998. PMID: 9623352 Review. Serbian.
-
Diagnosis and management of the antiphospholipid syndrome.Blood Rev. 2017 Nov;31(6):406-417. doi: 10.1016/j.blre.2017.07.006. Epub 2017 Jul 30. Blood Rev. 2017. PMID: 28784423 Free PMC article. Review.
Cited by
-
Pituitary-Adrenal Axis and Peripheral Immune Cell Profile in Long COVID.Biomedicines. 2024 Mar 5;12(3):581. doi: 10.3390/biomedicines12030581. Biomedicines. 2024. PMID: 38540194 Free PMC article.
-
Impaired innate and adaptive immune responses to BNT162b2 SARS-CoV-2 vaccination in systemic lupus erythematosus.JCI Insight. 2024 Mar 8;9(5):e176556. doi: 10.1172/jci.insight.176556. JCI Insight. 2024. PMID: 38456511 Free PMC article.
-
Antiphospholipid antibodies in patients with COVID-19: a systematic review and meta-analysis.J Thromb Thrombolysis. 2025 Jul 5. doi: 10.1007/s11239-025-03116-z. Online ahead of print. J Thromb Thrombolysis. 2025. PMID: 40617907 Review.
References
-
- Hui D.S., Iazhar E., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. - DOI - PMC - PubMed
-
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. - DOI - PMC - PubMed
-
- Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C.D., Ageno W., Madjid M., Guo Y., et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

