In Vitro Study of a Novel Vibrio alginolyticus-Based Collagenase for Future Medical Application
- PMID: 37626834
- PMCID: PMC10453626
- DOI: 10.3390/cells12162025
In Vitro Study of a Novel Vibrio alginolyticus-Based Collagenase for Future Medical Application
Abstract
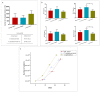
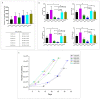
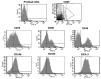
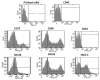
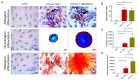
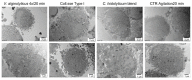
Mesenchymal stem cells extracted from adipose tissue are particularly promising given the ease of harvest by standard liposuction and reduced donor site morbidity. This study proposes a novel enzymatic method for isolating stem cells using Vibrio alginolyticus collagenase, obtaining a high-quality product in a reduced time. Initially, the enzyme concentration and incubation time were studied by comparing cellular yield, proliferation, and clonogenic capacities. The optimized protocol was phenotypically characterized, and its ability to differentiate in the mesodermal lineages was evaluated. Subsequently, that protocol was compared with two Clostridium histolyticum-based collagenases, and other tests for cellular integrity were performed to evaluate the enzyme's effect on expanded cells. The best results showed that using a concentration of 3.6 mg/mL Vibrio alginolyticus collagenase allows extracting stem cells from adipose tissue after 20 min of enzymatic reaction like those obtained with Clostridium histolyticum-based collagenases after 45 min. Moreover, the extracted cells with Vibrio alginolyticus collagenase presented the phenotypic characteristics of stem cells that remain after culture conditions. Finally, it was seen that Vibrio alginolyticus collagenase does not reduce the vitality of expanded cells as Clostridium histolyticum-based collagenase does. These findings suggest that Vibrio alginolyticus collagenase has great potential in regenerative medicine, given its degradation selectivity by protecting vital structures for tissue restructuration.
Keywords: Vibrio alginolyticus; adipose tissue; collagenase; disaggregation; regenerative medicine.
Conflict of interest statement
Authors Michele Caputo and Christian Cuppari are employees of Fidia Pharmaceutical, who provided the materials to be analyzed. The results described are entirely those seen by the authors and do not necessarily reflect the interest of Fidia Pharmaceutical. The remaining authors have no conflict of interest to declare.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

