Neuronal atg1 Coordinates Autophagy Induction and Physiological Adaptations to Balance mTORC1 Signalling
- PMID: 37626835
- PMCID: PMC10453232
- DOI: 10.3390/cells12162024
Neuronal atg1 Coordinates Autophagy Induction and Physiological Adaptations to Balance mTORC1 Signalling
Abstract
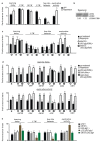
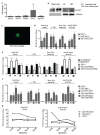
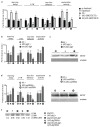
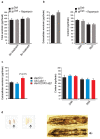
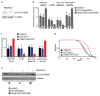
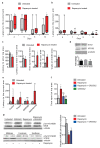
The mTORC1 nutrient-sensing pathway integrates metabolic and endocrine signals into the brain to evoke physiological responses to food deprivation, such as autophagy. Nevertheless, the impact of neuronal mTORC1 activity on neuronal circuits and organismal metabolism remains obscure. Here, we show that mTORC1 inhibition acutely perturbs serotonergic neurotransmission via proteostatic alterations evoked by the autophagy inducer atg1. Neuronal ATG1 alters the intracellular localization of the serotonin transporter, which increases the extracellular serotonin and stimulates the 5HTR7 postsynaptic receptor. 5HTR7 enhances food-searching behaviour and ecdysone-induced catabolism in Drosophila. Along similar lines, the pharmacological inhibition of mTORC1 in zebrafish also stimulates food-searching behaviour via serotonergic activity. These effects occur in parallel with neuronal autophagy induction, irrespective of the autophagic activity and the protein synthesis reduction. In addition, ectopic neuronal atg1 expression enhances catabolism via insulin pathway downregulation, impedes peptidergic secretion, and activates non-cell autonomous cAMP/PKA. The above exert diverse systemic effects on organismal metabolism, development, melanisation, and longevity. We conclude that neuronal atg1 aligns neuronal autophagy induction with distinct physiological modulations, to orchestrate a coordinated physiological response against reduced mTORC1 activity.
Keywords: 5HTR7 receptor; ATG1; ageing; autophagy; behaviour; cAMP/PKA; ecdysone; longevity; mTORC1; metabolism; serotonin transporter.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



Similar articles
-
Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death.Curr Biol. 2007 Jan 9;17(1):1-11. doi: 10.1016/j.cub.2006.10.053. Curr Biol. 2007. PMID: 17208179 Free PMC article.
-
Atg1-independent induction of autophagy by the Drosophila Ulk3 homolog, ADUK.FEBS J. 2016 Nov;283(21):3889-3897. doi: 10.1111/febs.13906. Epub 2016 Oct 11. FEBS J. 2016. PMID: 27717182 Free PMC article.
-
The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy.Proc Natl Acad Sci U S A. 2009 Oct 6;106(40):17049-54. doi: 10.1073/pnas.0903316106. Epub 2009 Sep 21. Proc Natl Acad Sci U S A. 2009. PMID: 19805182 Free PMC article.
-
mTORC1 and Nutrient Homeostasis: The Central Role of the Lysosome.Int J Mol Sci. 2018 Mar 12;19(3):818. doi: 10.3390/ijms19030818. Int J Mol Sci. 2018. PMID: 29534520 Free PMC article. Review.
-
Early signalling events of autophagy.Essays Biochem. 2013;55:1-15. doi: 10.1042/bse0550001. Essays Biochem. 2013. PMID: 24070467 Review.
References
-
- Villanueva E.C., Munzberg H., Cota D., Leshan R.L., Kopp K., Ishida-Takahashi R., Jones J.C., Fingar D.C., Seeley R.J., Myers M.G., Jr. Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology. 2009;150:4541–4551. doi: 10.1210/en.2009-0642. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

