Sexual Dimorphism in the Mechanism of Pain Central Sensitization
- PMID: 37626838
- PMCID: PMC10453375
- DOI: 10.3390/cells12162028
Sexual Dimorphism in the Mechanism of Pain Central Sensitization
Abstract
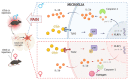
It has long been recognized that men and women have different degrees of susceptibility to chronic pain. Greater recognition of the sexual dimorphism in chronic pain has resulted in increasing numbers of both clinical and preclinical studies that have identified factors and mechanisms underlying sex differences in pain sensitization. Here, we review sexually dimorphic pain phenotypes in various research animal models and factors involved in the sex difference in pain phenotypes. We further discuss putative mechanisms for the sexual dimorphism in pain sensitization, which involves sex hormones, spinal cord microglia, and peripheral immune cells. Elucidating the sexually dimorphic mechanism of pain sensitization may provide important clinical implications and aid the development of sex-specific therapeutic strategies to treat chronic pain.
Keywords: central sensitization; microglia; neuropathic pain; sexual dimorphism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Treede R.-D., Rief W., Barke A., Aziz Q., Bennett M.I., Benoliel R., Cohen M., Evers S., Finnerup N.B., First M.B., et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11) Pain. 2019;160:19–27. doi: 10.1097/j.pain.0000000000001384. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

