Reduction of Ca2+ Entry by a Specific Block of KCa3.1 Channels Optimizes Cytotoxic Activity of NK Cells against T-ALL Jurkat Cells
- PMID: 37626875
- PMCID: PMC10453324
- DOI: 10.3390/cells12162065
Reduction of Ca2+ Entry by a Specific Block of KCa3.1 Channels Optimizes Cytotoxic Activity of NK Cells against T-ALL Jurkat Cells
Abstract
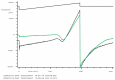
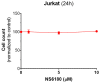
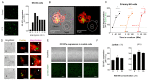
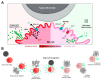
Degranulation mediated killing mechanism by NK cells is dependent on store-operated Ca2+ entry (SOCE) and has optimum at moderate intracellular Ca2+ elevations so that partial block of SOCE optimizes the killing process. In this study, we tested the effect of the selective blocker of KCa3.1 channel NS6180 on SOCE and the killing efficiency of NK cells from healthy donors and NK-92 cells against T-ALL cell line Jurkat. Patch-clamp analysis showed that only one-quarter of resting NK cells functionally express KCa3.1 current, which increases 3-fold after activation by interleukins 15 and 2. Nevertheless, blockage of KCa3.1 significantly reduced SOCE and intracellular Ca2+ rise induced by IL-15 or target cell recognition. NS6180 (1 μM) decreased NK degranulation at zero time of coculture with Jurkat cells but already after 1 h, the degranulation reached the same level as in the control. Monitoring of target cell death by flow cytometry and confocal microscopy demonstrated that NS6180 significantly improved the killing ability of NK cells after 1 h in coculture with Jurkat cells and increased the Jurkat cell fraction with apoptotic and necrotic markers. Our data evidence a strong dependence of SOCE on KCa3.1 activity in NK cells and that KCa3.1 specific block can improve NK cytotoxicity.
Keywords: Jurkat cells; KCa3.1 channel; NK cells; NK-92; NK-mediated killing; acute lymphoblastic leukemia; intracellular calcium; store-operated calcium entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Blocking KCa3.1 channels increases tumor cell killing by a subpopulation of human natural killer lymphocytes.PLoS One. 2013 Oct 11;8(10):e76740. doi: 10.1371/journal.pone.0076740. eCollection 2013. PLoS One. 2013. PMID: 24146918 Free PMC article.
-
Calcium-dependent potassium channels control proliferation of cardiac progenitor cells and bone marrow-derived mesenchymal stem cells.J Physiol. 2018 Jun;596(12):2359-2379. doi: 10.1113/JP275388. Epub 2018 May 5. J Physiol. 2018. PMID: 29574723 Free PMC article.
-
Inhibition of KCa3.1 by depolarisation and 2-aminoethoxydiphenyl borate (2-APB) during Ca²⁺ release activated Ca²⁺ (CRAC) entry in human erythroleukemia (HEL) cells: Implications for the interpretation of 2-APB inhibition of CRAC entry.Cell Calcium. 2015 Feb;57(2):76-88. doi: 10.1016/j.ceca.2014.12.009. Epub 2014 Dec 23. Cell Calcium. 2015. PMID: 25601026
-
A calcium optimum for cytotoxic T lymphocyte and natural killer cell cytotoxicity.J Physiol. 2018 Jul;596(14):2681-2698. doi: 10.1113/JP274964. Epub 2018 Mar 12. J Physiol. 2018. PMID: 29368348 Free PMC article.
-
Calcium, cancer and killing: the role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells.Biochim Biophys Acta. 2013 Jul;1833(7):1603-11. doi: 10.1016/j.bbamcr.2012.11.016. Epub 2012 Dec 3. Biochim Biophys Acta. 2013. PMID: 23220009 Review.
Cited by
-
Calcium channels as pharmacological targets for cancer therapy.Clin Exp Med. 2025 Mar 25;25(1):94. doi: 10.1007/s10238-025-01632-z. Clin Exp Med. 2025. PMID: 40131496 Free PMC article. Review.
-
Activity of Potassium Channels in CD8+ T Lymphocytes: Diagnostic and Prognostic Biomarker in Ovarian Cancer?Int J Mol Sci. 2024 Feb 6;25(4):1949. doi: 10.3390/ijms25041949. Int J Mol Sci. 2024. PMID: 38396628 Free PMC article.
-
Regulation of T Lymphocyte Functions through Calcium Signaling Modulation by Nootkatone.Int J Mol Sci. 2024 May 11;25(10):5240. doi: 10.3390/ijms25105240. Int J Mol Sci. 2024. PMID: 38791278 Free PMC article.
-
Kir6.1, a component of an ATP-sensitive potassium channel, regulates natural killer cell development.Front Immunol. 2024 Dec 2;15:1490250. doi: 10.3389/fimmu.2024.1490250. eCollection 2024. Front Immunol. 2024. PMID: 39687626 Free PMC article.
References
-
- Maul-Pavicic A., Chiang S.C.C., Rensing-Ehl A., Jessen B., Fauriat C., Wood S.M., Sjöqvist S., Hufnagel M., Bass T., Shamel W.W., et al. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cells lysis. Proc. Natl. Acad. Sci. USA. 2011;108:3324–3329. doi: 10.1073/pnas.1013285108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

