Enhanced Membrane Fluidization and Cholesterol Displacement by 1-Heptanol Inhibit Mast Cell Effector Functions
- PMID: 37626879
- PMCID: PMC10453462
- DOI: 10.3390/cells12162069
Enhanced Membrane Fluidization and Cholesterol Displacement by 1-Heptanol Inhibit Mast Cell Effector Functions
Abstract
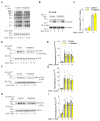
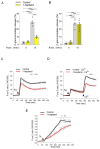
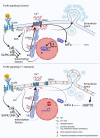
Signal transduction by the high-affinity IgE receptor (FcεRI) depends on membrane lipid and protein compartmentalization. Recently published data show that cells treated with 1-heptanol, a cell membrane fluidizer, exhibit changes in membrane properties. However, the functional consequences of 1-heptanol-induced changes on mast cell signaling are unknown. This study shows that short-term exposure to 1-heptanol reduces membrane thermal stability and dysregulates mast cell signaling at multiple levels. Cells treated with 1-heptanol exhibited increased lateral mobility and decreased internalization of the FcεRI. However, this did not affect the initial phosphorylation of the FcεRI-β chain and components of the SYK/LAT1/PLCγ1 signaling pathway after antigen activation. In contrast, 1-heptanol inhibited SAPK/JNK phosphorylation and effector functions such as calcium response, degranulation, and cytokine production. Membrane hyperfluidization induced a heat shock-like response via increased expression of the heat shock protein 70, increased lateral diffusion of ORAI1-mCherry, and unsatisfactory performance of STIM1-ORAI1 coupling, as determined by flow-FRET. Furthermore, 1-heptanol inhibited the antigen-induced production of reactive oxygen species and potentiated stress-induced plasma membrane permeability by interfering with heat shock protein 70 activity. The combined data suggest that 1-heptanol-mediated membrane fluidization does not interfere with the earliest biochemical steps of FcεRI signaling, such as phosphorylation of the FcεRI-β chain and components of the SYK/LAT/PLCγ1 signaling pathway, instead inhibiting the FcεRI internalization and mast cell effector functions, including degranulation and cytokine production.
Keywords: FRAP; FcεRI signaling; STIM1-ORAI1 coupling; alkanol; flow-FRET; heat shock response; membrane fluidizer; store-operated calcium entry.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures









Similar articles
-
Ethanol Inhibits High-Affinity Immunoglobulin E Receptor (FcεRI) Signaling in Mast Cells by Suppressing the Function of FcεRI-Cholesterol Signalosome.PLoS One. 2015 Dec 14;10(12):e0144596. doi: 10.1371/journal.pone.0144596. eCollection 2015. PLoS One. 2015. PMID: 26658290 Free PMC article.
-
The FcεRIβ homologue, MS4A4A, promotes FcεRI signal transduction and store-operated Ca2+ entry in human mast cells.Cell Signal. 2020 Jul;71:109617. doi: 10.1016/j.cellsig.2020.109617. Epub 2020 Mar 30. Cell Signal. 2020. PMID: 32240745 Free PMC article.
-
Pentacyclic triterpenoid ursolic acid interferes with mast cell activation via a lipid-centric mechanism affecting FcεRI signalosome functions.J Biol Chem. 2022 Nov;298(11):102497. doi: 10.1016/j.jbc.2022.102497. Epub 2022 Sep 15. J Biol Chem. 2022. PMID: 36115460 Free PMC article.
-
A perspective: regulation of IgE receptor-mediated mast cell responses by a LAT-organized plasma membrane-localized signaling complex.Int Arch Allergy Immunol. 2001 Jan-Mar;124(1-3):137-41. doi: 10.1159/000053692. Int Arch Allergy Immunol. 2001. PMID: 11306950 Review.
-
FcepsilonRI signaling observed from the inside of the mast cell membrane.Mol Immunol. 2002 Sep;38(16-18):1259-68. doi: 10.1016/s0161-5890(02)00073-1. Mol Immunol. 2002. PMID: 12217393 Review.
References
-
- Bag N., Wagenknecht-wiesner A., Lee A., Shi S.M., Holowka D.A., Baird B.A. Lipid-based and protein-based interactions synergize transmembrane signaling stimulated by antigen clustering of IgE receptors. Proc. Natl. Acad. Sci. USA. 2021;118:e2026583118. doi: 10.1073/pnas.2026583118. - DOI - PMC - PubMed
-
- Kovárová M., Tolar P., Arudchandran R., Dráberová L., Rivera J., Dráber P. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcε receptor I aggregation. Mol. Cell Biol. 2001;21:8318–8328. doi: 10.1128/MCB.21.24.8318-8328.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

