Catch of the Day: New Serum Amyloid A (SAA) Antibody Is a Valuable Tool to Study Fish Health in Salmonids
- PMID: 37626907
- PMCID: PMC10453338
- DOI: 10.3390/cells12162097
Catch of the Day: New Serum Amyloid A (SAA) Antibody Is a Valuable Tool to Study Fish Health in Salmonids
Abstract
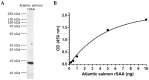
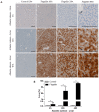
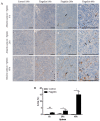
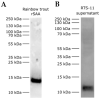
Serum amyloid A (SAA) proteins belong to a family of acute-phase reactants, playing an integral role in defending the organism from pathological damage. Despite a wealth of data on the regulation of SAA transcripts in teleosts, there is only limited information on these proteins' abundance in fish. The aim of this study is to characterise SAA protein levels in salmonids using a newly developed antibody specific to salmonid SAA. The salmonid SAA antibody detected SAA and accurately discriminated between stimulated and control specimens from rainbow trout macrophage cell line (RTS-11) in vitro, as well as rainbow trout challenged with Aeromonas salmonicida- or flagellin-stimulated Atlantic salmon in vivo. The presence of SAA protein was analysed in RTS-11 cell line supernatants, liver, and spleen samples using ELISA, immunoblotting, and immunohistochemistry. This study is the first to characterise SAA protein levels in salmonids in vivo and in vitro. The newly developed salmonid SAA antibody was able to discriminate between stimulated and unstimulated specimens, showing that it can be used to study the acute-phase response in salmonids with the potential to be further developed into assays to monitor and evaluate health in wild and farmed fish.
Keywords: RTS-11 cell line; SAA antibody; SAA recombinant protein; fish health; research tools; salmonids; serum amyloid A (SAA).
Conflict of interest statement
R.B. is a knowledge transfer partnership (KTP) associate funded by Innovate UK and VAL. A.A. (Ayham Alnabulsi), A.A. (Abdo Alnabulsi), and A.W. are employees of VAL.
Figures


References
-
- Jorgensen J.B., Lunde H., Jensen L., Whitehead A.S., Robertsen B. Serum amyloid A transcription in Atlantic salmon (Salmo salar L.) hepatocytes is enhanced by stimulation with macrophage factors, recombinant human IL-1 beta, IL-6 and TNF alpha or bacterial lipopolysaccharide. Dev. Comp. Immunol. 2000;24:553–563. doi: 10.1016/S0145-305X(00)00022-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

