Effect of Expansion Media on Functional Characteristics of Bone Marrow-Derived Mesenchymal Stromal Cells
- PMID: 37626914
- PMCID: PMC10453497
- DOI: 10.3390/cells12162105
Effect of Expansion Media on Functional Characteristics of Bone Marrow-Derived Mesenchymal Stromal Cells
Abstract
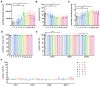
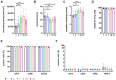
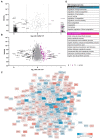
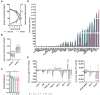
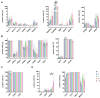
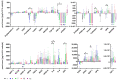
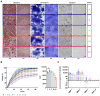
The therapeutic efficacy of mesenchymal stromal cells (MSCs) has been shown to rely on their immunomodulatory and regenerative properties. In order to obtain sufficient numbers of cells for clinical applications, MSCs have to be expanded ex vivo. Expansion media with xenogeneic-free (XF) growth-promoting supplements like human platelet lysate (PL) or serum- and xenogeneic-free (SF/XF) formulations have been established as safe and efficient, and both groups provide different beneficial qualities. In this study, MSCs were expanded in XF or SF/XF media as well as in mixtures thereof. MSCs cultured in these media were analyzed for phenotypic and functional properties. MSC expansion was optimal with SF/XF conditions when PL was present. Metabolic patterns, consumption of growth factors, and secretome of MSCs differed depending on the type and concentration of supplement. The lactate per glucose yield increased along with a higher proportion of PL. Many factors in the supernatant of cultured MSCs showed distinct patterns depending on the supplement (e.g., FGF-2, TGFβ, and insulin only in PL-expanded MSC, and leptin, sCD40L PDGF-AA only in SF/XF-expanded MSC). This also resulted in changes in cell characteristics like migratory potential. These findings support current approaches where growth media may be utilized for priming MSCs for specific therapeutic applications.
Keywords: media; mesenchymal stem cells; mesenchymal stromal cells; platelet lysate; serum-free; xenogeneic-free.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Friedenstein A.J., Gorskaja J.F., Kulagina N.N. Fibroblast Precursors in Normal and Irradiated Mouse Hematopoietic Organs. Exp. Hematol. 1976;4:267–274. - PubMed
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
-
- Soler R., Orozco L., Munar A., Huguet M., Lopez R., Vives J., Coll R., Codinach M., Garcia-Lopez J. Final Results of a Phase I-Ii Trial Using Ex Vivo Expanded Autologous Mesenchymal Stromal Cells for the Treatment of Osteoarthritis of the Knee Confirming Safety and Suggesting Cartilage Regeneration. Knee. 2016;23:647–654. doi: 10.1016/j.knee.2015.08.013. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

