Glucocorticoids and Their Receptor Isoforms: Roles in Female Reproduction, Pregnancy, and Foetal Development
- PMID: 37626990
- PMCID: PMC10452123
- DOI: 10.3390/biology12081104
Glucocorticoids and Their Receptor Isoforms: Roles in Female Reproduction, Pregnancy, and Foetal Development
Abstract
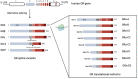
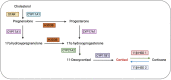
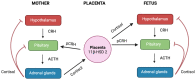
Alterations in the hypothalamic-pituitary-adrenal (HPA) axis and associated changes in circulating levels of glucocorticoids are integral to an organism's response to stressful stimuli. Glucocorticoids acting via glucocorticoid receptors (GRs) play a role in fertility, reproduction, placental function, and foetal development. GRs are ubiquitously expressed throughout the female reproductive system and regulate normal reproductive function. Stress-induced glucocorticoids have been shown to inhibit reproduction and affect female gonadal function by suppressing the hypothalamic-pituitary-gonadal (HPG) axis at each level. Furthermore, during pregnancy, a mother's exposure to prenatal stress or external glucocorticoids can result in long-lasting alterations to the foetal HPA and neuroendocrine function. Several GR isoforms generated via alternative splicing or translation initiation from the GR gene have been identified in the mammalian ovary and uterus. The GR isoforms identified include the splice variants, GRα and GRβ, and GRγ and GR-P. Glucocorticoids can exert both stimulatory and inhibitory effects and both pro- and anti-inflammatory functions in the ovary, in vitro. In the placenta, thirteen GR isoforms have been identified in humans, guinea pigs, sheep, rats, and mice, indicating they are conserved across species and may be important in mediating a differential response to stress. Distinctive responses to glucocorticoids, differential birth outcomes in pregnancy complications, and sex-based variations in the response to stress could all potentially be dependent on a particular GR expression pattern. This comprehensive review provides an overview of the structure and function of the GR in relation to female fertility and reproduction and discusses the changes in the GR and glucocorticoid signalling during pregnancy. To generate this overview, an extensive non-systematic literature search was conducted across multiple databases, including PubMed, Web of Science, and Google Scholar, with a focus on original research articles, meta-analyses, and previous review papers addressing the subject. This review integrates the current understanding of GR variants and their roles in glucocorticoid signalling, reproduction, placental function, and foetal growth.
Keywords: glucocorticoid receptor; pregnancy; reproduction; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

