Effect of Mesenchymal Stem Cells Overexpressing BMP-9 Primed with Hypoxia on BMP Targets, Osteoblast Differentiation and Bone Repair
- PMID: 37627031
- PMCID: PMC10452403
- DOI: 10.3390/biology12081147
Effect of Mesenchymal Stem Cells Overexpressing BMP-9 Primed with Hypoxia on BMP Targets, Osteoblast Differentiation and Bone Repair
Abstract
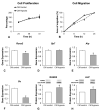
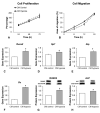
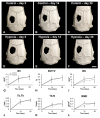
Bone formation is driven by many signaling molecules including bone morphogenetic protein 9 (BMP-9) and hypoxia-inducible factor 1-alpha (HIF-1α). We demonstrated that cell therapy using mesenchymal stem cells (MSCs) overexpressing BMP-9 (MSCs+BMP-9) enhances bone formation in calvarial defects. Here, the effect of hypoxia on BMP components and targets of MSCs+BMP-9 and of these hypoxia-primed cells on osteoblast differentiation and bone repair was evaluated. Hypoxia was induced with cobalt chloride (CoCl2) in MSCs+BMP-9, and the expression of BMP components and targets was evaluated. The paracrine effects of hypoxia-primed MSCs+BMP-9 on cell viability and migration and osteoblast differentiation were evaluated using conditioned medium. The bone formation induced by hypoxia-primed MSCs+BMP-9 directly injected into rat calvarial defects was also evaluated. The results demonstrated that hypoxia regulated BMP components and targets without affecting BMP-9 amount and that the conditioned medium generated under hypoxia favored cell migration and osteoblast differentiation. Hypoxia-primed MSCs+BMP-9 did not increase bone repair compared with control MSCs+BMP-9. Thus, despite the lack of effect of hypoxia on bone formation, the enhancement of cell migration and osteoblast differentiation opens windows for further investigations on approaches to modulate the BMP-9-HIF-1α circuit in the context of cell-based therapies to induce bone regeneration.
Keywords: BMP-9; HIF-1α; bone; cell therapy; hypoxia; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotente mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
-
- Freitas G.P., Lopes H.B., Souza A.T.P., Oliveira P.G.F.P., Almeida A.L.G., Coelho P.G., Ferreira F.U., Covas D.T., Beloti M.M., Rosa A.L. Effect of cell therapy with osteoblasts differentiated from bone marrow or adipose tissue stromal cells on bone repair. Regen. Med. 2019;14:1107–1119. doi: 10.2217/rme-2019-0036. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

