In Patients Treated by Selective Internal Radiotherapy, Cellular In Vitro Immune Function Is Predictive of Survival
- PMID: 37627082
- PMCID: PMC10452121
- DOI: 10.3390/cancers15164055
In Patients Treated by Selective Internal Radiotherapy, Cellular In Vitro Immune Function Is Predictive of Survival
Abstract
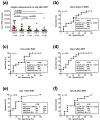
In patients with liver malignancies, the cellular immune function was impaired in vitro after selective internal radiotherapy (SIRT). Because immunosuppression varied substantially, in the current study, we investigated in 25 SIRT patients followed up for ten years whether the lymphocyte function was correlated with survival. Peripheral blood mononuclear cells were stimulated with four microbial antigens (tuberculin, tetanus toxoid, Candida albicans and CMV) before therapy and at four time points thereafter, and lymphocyte proliferation was determined by H3-thymidine uptake. The median sum of the responses to these four antigens decreased from 39,464 counts per minute (CPM) increment (range 1080-204,512) before therapy to a minimum of 700 CPM increment on day 7 after therapy (0-93,187, p < 0.0001). At all five time points, the median survival in patients with weaker responses was 2- to 3.5-fold shorter (p < 0.05). On day 7, the median survival in patients with responses below and above the cutoff of a 2 CPM increment was 185 and 523 days, respectively (χ2 = 9.4, p = 0.002). In conclusion, lymphocyte function could be a new predictor of treatment outcome after SIRT.
Keywords: ELISpot; immune checkpoint molecule; interferon-gamma; interleukin-2; lymphocyte proliferation; patient survival; selective internal radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Theelen W., Peulen H.M.U., Lalezari F., van der Noort V., de Vries J.F., Aerts J., Dumoulin D.W., Bahce I., Niemeijer A.N., de Langen A.J., et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

