Mitochondrial Peptide Humanin Facilitates Chemoresistance in Glioblastoma Cells
- PMID: 37627089
- PMCID: PMC10452904
- DOI: 10.3390/cancers15164061
Mitochondrial Peptide Humanin Facilitates Chemoresistance in Glioblastoma Cells
Abstract
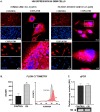
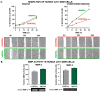
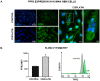
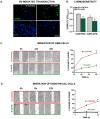
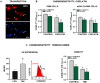
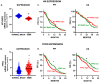
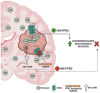
Humanin (HN) is a mitochondrial-derived peptide with robust cytoprotective effects in many cell types. Although the administration of HN analogs has been proposed to treat degenerative diseases, its role in the pathogenesis of cancer is poorly understood. Here, we evaluated whether HN affects the chemosensitivity of glioblastoma (GBM) cells. We found that chemotherapy upregulated HN expression in GBM cell lines and primary cultures derived from GBM biopsies. An HN analog (HNGF6A) boosted chemoresistance, increased the migration of GBM cells and improved their capacity to induce endothelial cell migration and proliferation. Chemotherapy also upregulated FPR2 expression, an HN membrane-bound receptor, and the HNGF6A cytoprotective effects were inhibited by an FPR2 receptor antagonist (WRW4). These effects were observed in glioma cells with heterogeneous genetic backgrounds, i.e., glioma cells with wild-type (wtIDH) and mutated (mIDH) isocitrate dehydrogenase. HN silencing using a baculoviral vector that encodes for a specific shRNA for HN (BV.shHN) reduced chemoresistance, and impaired the migration and proangiogenic capacity of GBM cells. Taken together, our findings suggest that HN boosts the hallmark characteristics of GBM, i.e., chemoresistance, migration and endothelial cell proliferation. Thus, strategies that inhibit the HN/FPR2 pathway may improve the response of GBM to standard therapy.
Keywords: FPR2; chemotherapy; glioblastoma; humanin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Chinot O.L., Barrie M., Fuentes S., Eudes N., Lancelot S., Metellus P., Muracciole X., Braguer D., Ouafik L., Martin P.M., et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J. Clin. Oncol. 2007;25:1470–1475. doi: 10.1200/JCO.2006.07.4807. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

