BRD4 Inhibition as a Strategy to Prolong the Response to Standard of Care in Estrogen Receptor-Positive Breast Cancer
- PMID: 37627092
- PMCID: PMC10452571
- DOI: 10.3390/cancers15164066
BRD4 Inhibition as a Strategy to Prolong the Response to Standard of Care in Estrogen Receptor-Positive Breast Cancer
Abstract
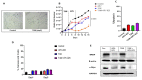
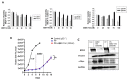
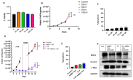
Breast cancer is the most commonly occurring malignancy in women and the second most common cause of cancer-related deaths. ER+ breast cancer constitutes approximately 70% of all breast cancer cases. The standard of care for ER+ breast cancer involves estrogen antagonists such as tamoxifen or fulvestrant in combination with CDK4/6 inhibitors such as palbociclib. However, these treatments are often not curative, with disease recurrence and metastasis being responsible for patient mortality. Overexpression of the epigenetic regulator, BRD4, has been shown to be a negative prognostic indicator in breast cancer, and BET family inhibitors such as ARV-825 and ABBV-744 have garnered interest for their potential to improve and prolong the response to current therapeutic strategies. The current work examined the potential of utilizing ARV-825 and ABBV-744 to increase the effectiveness of tamoxifen or fulvestrant plus palbociclib. ARV-825 was effective in both p53 wild-type (WT) breast tumor cells and in cells lacking functional p53 either alone or in combination with tamoxifen, while the effectiveness of ABBV-744 was limited to fulvestrant plus palbociclib in p53 WT cells. These differential effects may be related to the capacity to suppress c-Myc, a downstream target of BRD4.
Keywords: ABBV-744; ARV-825; BRD4; c-Myc; p53; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The BET inhibitor/degrader ARV-825 prolongs the growth arrest response to Fulvestrant + Palbociclib and suppresses proliferative recovery in ER-positive breast cancer.Front Oncol. 2023 Jan 18;12:966441. doi: 10.3389/fonc.2022.966441. eCollection 2022. Front Oncol. 2023. PMID: 36741704 Free PMC article.
-
MDM2 inhibition in combination with endocrine therapy and CDK4/6 inhibition for the treatment of ER-positive breast cancer.Breast Cancer Res. 2020 Aug 12;22(1):87. doi: 10.1186/s13058-020-01318-2. Breast Cancer Res. 2020. PMID: 32787886 Free PMC article.
-
Lasofoxifene as a potential treatment for therapy-resistant ER-positive metastatic breast cancer.Breast Cancer Res. 2021 May 12;23(1):54. doi: 10.1186/s13058-021-01431-w. Breast Cancer Res. 2021. PMID: 33980285 Free PMC article.
-
A Review of Fulvestrant in Breast Cancer.Oncol Ther. 2017;5(1):17-29. doi: 10.1007/s40487-017-0046-2. Epub 2017 May 8. Oncol Ther. 2017. PMID: 28680952 Free PMC article. Review.
-
Palbociclib: A Novel Cyclin-Dependent Kinase Inhibitor for Hormone Receptor-Positive Advanced Breast Cancer.Ann Pharmacother. 2015 Nov;49(11):1252-60. doi: 10.1177/1060028015602273. Epub 2015 Aug 31. Ann Pharmacother. 2015. PMID: 26324355 Free PMC article. Review.
Cited by
-
CDK4/6 inhibitors upregulate cIAP1/2, and Smac mimetic LCL161 enhances their antitumor effects in cholangiocarcinoma cells.Sci Rep. 2025 Feb 25;15(1):6826. doi: 10.1038/s41598-025-90997-y. Sci Rep. 2025. PMID: 40000765 Free PMC article.
-
Autophagy and senescence facilitate the development of antiestrogen resistance in ER positive breast cancer.Front Endocrinol (Lausanne). 2024 Mar 18;15:1298423. doi: 10.3389/fendo.2024.1298423. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38567308 Free PMC article. Review.
-
A Conversation with ChatGPT on Contentious Issues in Senescence and Cancer Research.Mol Pharmacol. 2024 Apr 17;105(5):313-327. doi: 10.1124/molpharm.124.000871. Mol Pharmacol. 2024. PMID: 38458774 Free PMC article.
-
ABBV-744 alleviates LPS-induced neuroinflammation via regulation of BATF2-IRF4-STAT1/3/5 axis.Acta Pharmacol Sin. 2024 Oct;45(10):2077-2091. doi: 10.1038/s41401-024-01318-4. Epub 2024 Jun 11. Acta Pharmacol Sin. 2024. PMID: 38862817
-
The common yet enigmatic activity of histone tail clipping.J Biol Chem. 2025 Jul;301(7):110239. doi: 10.1016/j.jbc.2025.110239. Epub 2025 May 15. J Biol Chem. 2025. PMID: 40381696 Free PMC article. Review.
References
-
- Kohler B.A., Sherman R.L., Howlader N., Jemal A., Ryerson A.B., Henry K.A., Boscoe F.P., Cronin K.A., Lake A., Noone A.M., et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J. Natl. Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

