Menin Maintains Cholesterol Content in Colorectal Cancer via Repression of LXR-Mediated Transcription
- PMID: 37627154
- PMCID: PMC10453013
- DOI: 10.3390/cancers15164126
Menin Maintains Cholesterol Content in Colorectal Cancer via Repression of LXR-Mediated Transcription
Abstract
Background and aims: Menin is a nuclear scaffold protein that regulates gene transcription in an oftentimes tissue-specific manner. Our previous work showed that menin is over-expressed in colorectal cancer (CRC); however, the full spectrum of menin function in colonic neoplasia remains unclear. Herein, we aimed to uncover novel menin-regulated pathways important for colorectal carcinogenesis.
Methods: RNA-Seq analysis identified that menin regulates LXR-target gene expressions in CRC cell lines. Isolated colonic epithelium from Men1f/f;Vil1-Cre and Men1f/f mice was used to validate the results in vivo. Cholesterol content was quantified via an enzymatic assay.
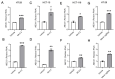
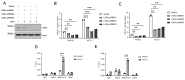
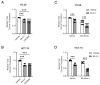
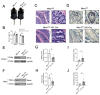
Results: RNA-Seq analysis in the HT-29 CRC cell line identified that menin inhibition upregulated LXR-target genes, specifically ABCG1 and ABCA1, with protein products that promote cellular cholesterol efflux. Similar results were noted across other CRC cell lines and with different methods of menin inhibition. Consistent with ABCG1 and ABCA1 upregulation, and similarly to LXR agonists, menin inhibition reduced the total cellular cholesterol in both HT-29 and HCT-15 cells. To confirm the effects of menin inhibition in vivo, we assessed Men1f/f;Vil1-Cre mice lacking menin expression in the colonic epithelium. Men1f/f;Vil1-Cre mice were found to have no distinct baseline phenotype compared to control Men1f/f mice. However, similarly to CRC cell lines, Men1f/f;Vil1-Cre mice showed an upregulation of Abcg1 and a reduction in total cellular cholesterol. Promoting cholesterol efflux, either via menin inhibition or LXR activation, was found to synergistically suppress CRC cell growth under cholesterol-depleted conditions and when administered concomitantly with small molecule EGFR inhibitors.
Conclusions: Menin represses the transcription of LXR-target genes, including ABCA1 and ABCG1 in the colonic epithelium and CRC. Menin inhibition conversely upregulates LXR-target genes and reduces total cellular cholesterol, demonstrating that menin inhibition may be an important mechanism for targeting cholesterol-dependent pathways in colorectal carcinogenesis.
Keywords: ABCA1; ABCG1; LXR; cholesterol; colorectal cancer; menin.
Conflict of interest statement
The authors have no conflict of interest.
Figures


Similar articles
-
Pioglitazone enhances cholesterol efflux from macrophages by increasing ABCA1/ABCG1 expressions via PPARγ/LXRα pathway: findings from in vitro and ex vivo studies.Atherosclerosis. 2011 Nov;219(1):141-50. doi: 10.1016/j.atherosclerosis.2011.07.113. Epub 2011 Aug 4. Atherosclerosis. 2011. PMID: 21862012
-
Discovery and implementation of transcriptional biomarkers of synthetic LXR agonists in peripheral blood cells.J Transl Med. 2008 Oct 16;6:59. doi: 10.1186/1479-5876-6-59. J Transl Med. 2008. PMID: 18925943 Free PMC article. Clinical Trial.
-
Poly(ADP-ribose) Polymerase 1 Represses Liver X Receptor-mediated ABCA1 Expression and Cholesterol Efflux in Macrophages.J Biol Chem. 2016 May 20;291(21):11172-84. doi: 10.1074/jbc.M116.726729. Epub 2016 Mar 29. J Biol Chem. 2016. PMID: 27026705 Free PMC article.
-
Twenty years of menin: emerging opportunities for restoration of transcriptional regulation in MEN1.Endocr Relat Cancer. 2017 Oct;24(10):T135-T145. doi: 10.1530/ERC-17-0281. Epub 2017 Aug 15. Endocr Relat Cancer. 2017. PMID: 28811299 Free PMC article. Review.
-
Multiple endocrine neoplasia type 1: a chromatin writer's block.J Intern Med. 2009 Jul;266(1):53-9. doi: 10.1111/j.1365-2796.2009.02115.x. J Intern Med. 2009. PMID: 19522825 Review.
Cited by
-
Menin in Cancer.Genes (Basel). 2024 Sep 21;15(9):1231. doi: 10.3390/genes15091231. Genes (Basel). 2024. PMID: 39336822 Free PMC article. Review.
-
Arachidonic acid released by PIK3CA mutant tumor cells triggers malignant transformation of colonic epithelium by inducing chromatin remodeling.Cell Rep Med. 2024 May 21;5(5):101510. doi: 10.1016/j.xcrm.2024.101510. Epub 2024 Apr 12. Cell Rep Med. 2024. PMID: 38614093 Free PMC article.
References
-
- Agarwal S.K., Kester M.B., Debelenko L.V., Heppner C., Emmert-Buck M.R., Skarulis M.C., Doppman J.L., Kim Y.S., Lubensky I.A., Zhuang Z., et al. Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum. Mol. Genet. 1997;6:1169–1175. doi: 10.1093/hmg/6.7.1169. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

