Mass Spectrometric Analysis of Cucurbitacins and Dihydrocucurbitacins from the Tuber of Citrullus naudinianus
- PMID: 37627233
- PMCID: PMC10452186
- DOI: 10.3390/biom13081168
Mass Spectrometric Analysis of Cucurbitacins and Dihydrocucurbitacins from the Tuber of Citrullus naudinianus
Abstract
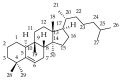
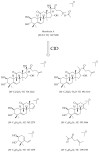
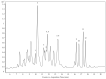
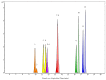
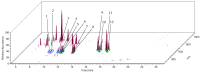
The vast pool of structurally and functionally distinct secondary metabolites (i.e., natural products (NPs)) is constantly being expanded, a process also driven by the rapid progress in the development of analytical techniques. Such NPs often show potent biological activities and are therefore prime candidates for drug development and medical applications. The ethyl acetate extract of the tuber of Citrullus naudinianus (C. naudinianus), an African melon with edible fruits and seeds, shows in vitro immunomodulatory activity presumably elicited by cucurbitacins that are known major constituents of this plant. Further potentially immunomodulatory cucurbitacins or cucurbitacin derivatives were assumed to be in the tuber. Given the typically high content of cucurbitacins with similar physicochemical features but often distinct bioactivities, an efficient and reliable separation process is a prerequisite for their detailed characterization and assessment in terms of bioactivity. We therefore developed a detection method to screen and differentiate cucurbitacins via high-performance liquid chromatography/quadrupole-time-of-flight tandem mass spectrometry (HPLC-QTOF-MS/MS). In order to confirm the identification, the fragmentation patterns of two cucurbitacins and one 23,24-dihydrocucurbitacin were also investigated. Six characteristic fragments were identified and three of them were employed for the identification of cucurbitacins and 23,24-dihydrocucurbitacins in the extract. As a result, in addition to eight previously reported cucurbitacins from this plant four distinct 23,24-dihydrocucurbitacins (B, D, E, and I) were putatively identified and newly found in the ethyl acetate extract of the tuber of C. naudinianus. The established methodology enables rapid and efficient LC-MS-based analysis and identification of cucurbitacins and 23,24-dihydrocucurbitacins in plant extracts.
Keywords: 23,24-dihydrocucurbitacins; Citrullus naudinianus; HPLC; MS; MS/MS; QTOF; cucurbitacins; orbitrap.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Grubben G.J.H., Denton O.A. Plant Resources of Tropical Africa 2, Vegetables. PROTA Foundation; Wageningen, The Netherlands: 2004. pp. 34–35.
-
- Rehm S., Enslin P.R., Meeuse A.D.J., Wessels J.H. Bitter principles of the cucurbitaceae. VII. †—The distribution of bitter principles in this plant family. J. Sci. Food Agric. 1957;8:679–686. doi: 10.1002/jsfa.2740081203. - DOI
-
- Miró M. Cucurbitacins and their pharmacological effects. Phytother. Res. 1995;9:159–168. doi: 10.1002/ptr.2650090302. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

