Chronic Hepatitis B Infection: New Approaches towards Cure
- PMID: 37627273
- PMCID: PMC10452112
- DOI: 10.3390/biom13081208
Chronic Hepatitis B Infection: New Approaches towards Cure
Abstract
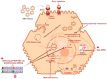
Chronic hepatitis B virus (HBV) infection leads to the development of cirrhosis and hepatocellular carcinoma. Lifelong treatment with nucleotides/nucleoside antiviral agents is effective at suppressing HBV replication, however, adherence to daily therapy can be challenging. This review discusses recent advances in the development of long-acting formulations for HBV treatment and prevention, which could potentially improve adherence. Promising new compounds that target distinct steps of the virus life cycle are summarized. In addition to treatments that suppress viral replication, curative strategies are focused on the elimination of covalently closed circular DNA and the inactivation of the integrated viral DNA from infected hepatocytes. We highlight promising long-acting antivirals and genome editing strategies for the elimination or deactivation of persistent viral DNA products in development.
Keywords: antivirals; chronic hepatitis B; gene editing; hepatitis B virus.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the collection, analyses, or interpretation of published literature, in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Sheena B.S., Hiebert L., Han H., Ippolito H., Abbasi-Kangevari M., Abbasi-Kangevari Z., Abbastabar H., Abdoli A., Ali H.A., Adane M.M., et al. Global, regional, and national burden of hepatitis B, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022;7:796–829. doi: 10.1016/S2468-1253(22)00124-8. - DOI - PMC - PubMed
-
- WHO. World Health Organization Fact Sheets. Hepatitis B. [(accessed on 3 January 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
-
- Weng M.K., Doshani M., Khan M.A., Frey S., Ault K., Moore K.L., Hall E.W., Morgan R.L., Campos-Outcalt D., Wester C., et al. Universal Hepatitis B Vaccination in Adults Aged 19–59 Years: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:477–483. doi: 10.15585/mmwr.mm7113a1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

