Autophagy/Mitophagy in Airway Diseases: Impact of Oxidative Stress on Epithelial Cells
- PMID: 37627282
- PMCID: PMC10452925
- DOI: 10.3390/biom13081217
Autophagy/Mitophagy in Airway Diseases: Impact of Oxidative Stress on Epithelial Cells
Abstract
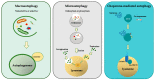
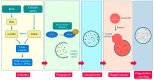
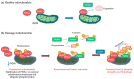
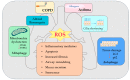
Autophagy is the key process by which the cell degrades parts of itself within the lysosomes. It maintains cell survival and homeostasis by removing molecules (particularly proteins), subcellular organelles, damaged cytoplasmic macromolecules, and by recycling the degradation products. The selective removal or degradation of mitochondria is a particular type of autophagy called mitophagy. Various forms of cellular stress (oxidative stress (OS), hypoxia, pathogen infections) affect autophagy by inducing free radicals and reactive oxygen species (ROS) formation to promote the antioxidant response. Dysfunctional mechanisms of autophagy have been found in different respiratory diseases such as chronic obstructive lung disease (COPD) and asthma, involving epithelial cells. Several existing clinically approved drugs may modulate autophagy to varying extents. However, these drugs are nonspecific and not currently utilized to manipulate autophagy in airway diseases. In this review, we provide an overview of different autophagic pathways with particular attention on the dysfunctional mechanisms of autophagy in the epithelial cells during asthma and COPD. Our aim is to further deepen and disclose the research in this direction to stimulate the develop of new and selective drugs to regulate autophagy for asthma and COPD treatment.
Keywords: COPD; asthma; autophagy; lung disease; mitophagy; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vasarmidi E., Sarantoulaki S., Trachalaki A., Margaritopoulos G., Bibaki E., Spandidos D.A., Tzanakis N., Antoniou K. Investigation of key autophagy-and mitophagy-related proteins and gene expression in BALF cells from patients with IPF and RA-ILD. Mol. Med. Rep. 2018;18:3891–3897. doi: 10.3892/mmr.2018.9356. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

