Cytochalasans and Their Impact on Actin Filament Remodeling
- PMID: 37627312
- PMCID: PMC10452583
- DOI: 10.3390/biom13081247
Cytochalasans and Their Impact on Actin Filament Remodeling
Erratum in
-
Correction: Lambert et al. Cytochalasans and Their Impact on Actin Filament Remodeling. Biomolecules 2023, 13, 1247.Biomolecules. 2025 Jul 16;15(7):1030. doi: 10.3390/biom15071030. Biomolecules. 2025. PMID: 40723926 Free PMC article.
Abstract
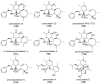
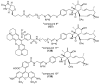
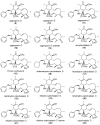
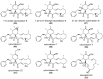
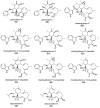
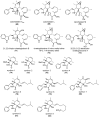
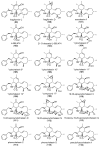
The eukaryotic actin cytoskeleton comprises the protein itself in its monomeric and filamentous forms, G- and F-actin, as well as multiple interaction partners (actin-binding proteins, ABPs). This gives rise to a temporally and spatially controlled, dynamic network, eliciting a plethora of motility-associated processes. To interfere with the complex inter- and intracellular interactions the actin cytoskeleton confers, small molecular inhibitors have been used, foremost of all to study the relevance of actin filaments and their turnover for various cellular processes. The most prominent inhibitors act by, e.g., sequestering monomers or by interfering with the polymerization of new filaments and the elongation of existing filaments. Among these inhibitors used as tool compounds are the cytochalasans, fungal secondary metabolites known for decades and exploited for their F-actin polymerization inhibitory capabilities. In spite of their application as tool compounds for decades, comprehensive data are lacking that explain (i) how the structural deviances of the more than 400 cytochalasans described to date influence their bioactivity mechanistically and (ii) how the intricate network of ABPs reacts (or adapts) to cytochalasan binding. This review thus aims to summarize the information available concerning the structural features of cytochalasans and their influence on the described activities on cell morphology and actin cytoskeleton organization in eukaryotic cells.
Keywords: actin binding proteins; actin inhibitors; chemo-diversity; eukaryotic actin cytoskeleton; secondary metabolites; structure–activity relationship.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Comprehensive Cell Biological Investigation of Cytochalasin B Derivatives with Distinct Activities on the Actin Network.J Nat Prod. 2024 Oct 25;87(10):2421-2431. doi: 10.1021/acs.jnatprod.4c00676. Epub 2024 Oct 11. J Nat Prod. 2024. PMID: 39392628 Free PMC article.
-
Xylariaides A and B, novel cytochalasans with a unique 5/6/5/3 ring system from a soil fungus Xylaria sp. Y01.Nat Prod Bioprospect. 2025 Apr 7;15(1):23. doi: 10.1007/s13659-025-00507-w. Nat Prod Bioprospect. 2025. PMID: 40192972 Free PMC article.
-
Correction: Lambert et al. Cytochalasans and Their Impact on Actin Filament Remodeling. Biomolecules 2023, 13, 1247.Biomolecules. 2025 Jul 16;15(7):1030. doi: 10.3390/biom15071030. Biomolecules. 2025. PMID: 40723926 Free PMC article.
-
Development of a microarray based telomerase binding assay reveals unusual binding of a cytochalasin derivative.Sci Rep. 2025 Jun 4;15(1):19515. doi: 10.1038/s41598-025-00230-z. Sci Rep. 2025. PMID: 40467649 Free PMC article.
-
Insights into Natural Products from Marine-Derived Fungi with Antimycobacterial Properties: Opportunities and Challenges.Mar Drugs. 2025 Jul 3;23(7):279. doi: 10.3390/md23070279. Mar Drugs. 2025. PMID: 40710504 Free PMC article. Review.
References
-
- Chepkirui C., Stadler M. The genus Diaporthe: A rich source of diverse and bioactive metabolites. Mycol. Prog. 2017;16:477–494. doi: 10.1007/s11557-017-1288-y. - DOI
-
- Charria-Girón E., Surup F., Marin-Felix Y. Diversity of biologically active secondary metabolites in the ascomycete order Sordariales. Mycol. Prog. 2022;21:43. doi: 10.1007/s11557-022-01775-3. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

