Brilliant Cresyl Blue Negative Oocytes Show a Reduced Competence for Embryo Development after In Vitro Fertilisation with Sperm Exposed to Oxidative Stress
- PMID: 37627412
- PMCID: PMC10451622
- DOI: 10.3390/ani13162621
Brilliant Cresyl Blue Negative Oocytes Show a Reduced Competence for Embryo Development after In Vitro Fertilisation with Sperm Exposed to Oxidative Stress
Abstract
The extent of oxidative damage transferred by the damaged sperm to the progeny is likely to be limited by the oocyte's repair and antioxidative capacity. We aimed to assess the association between Brilliant Cresyl Blue (BCB) staining in oocytes and their competence for embryo development after in vitro fertilisation (IVF) with damaged sperm. For this purpose, bovine sperm were incubated without (non-oxidised sperm, NOX S) or with 100 µM H2O2 (oxidised sperm, OX S) and were used to fertilise in-vitro-matured bovine oocytes (BCB-pos./BCB-neg.). Unstained oocytes served as controls (US). Development was assessed at 30, 46, 60 h and on Days (D) 7 and 8 after IVF. Total cell number and apoptotic index were analysed in D7 blastocysts. BCB-neg. oocytes showed lower cleavage rates and blastocyst rates than unstained oocytes after IVF with NOX S (p < 0.05). They showed the highest reduction in D7 blastocyst rate upon fertilisation with OX S and showed a delayed embryo development at 46 and 60 h after IVF compared to embryos produced with NOX S (p < 0.05). Total cell number in blastocysts produced with BCB-neg. oocytes was lower (p < 0.05) in the embryos produced with OX S than in embryos after IVF with NOX S. In conclusion, BCB-neg. oocytes have a lower competence to support embryo development after in vitro fertilisation with oxidised sperm.
Keywords: DNA damage; oocyte quality; sperm quality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

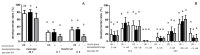

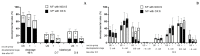
Similar articles
-
Bovine non-competent oocytes (BCB-) negatively impact the capacity of competent (BCB+) oocytes to undergo in vitro maturation, fertilisation and embryonic development.Zygote. 2016 Apr;24(2):245-51. doi: 10.1017/S0967199415000118. Epub 2015 May 6. Zygote. 2016. PMID: 25943119
-
Effect of oocyte quality on blastocyst development after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in a sheep model.Fertil Steril. 2012 Apr;97(4):1004-8. doi: 10.1016/j.fertnstert.2011.12.043. Epub 2012 Jan 21. Fertil Steril. 2012. PMID: 22265000
-
Developmental competence of prepubertal goat oocytes selected with brilliant cresyl blue and matured with cysteamine supplementation.Reprod Nutr Dev. 2003 Mar-Apr;43(2):179-87. doi: 10.1051/rnd:2003012. Reprod Nutr Dev. 2003. PMID: 12956317
-
The utility of Brilliant Cresyl Blue (BCB) staining of mammalian oocytes used for in vitro embryo production (IVP).Reprod Biol. 2013 Sep;13(3):177-83. doi: 10.1016/j.repbio.2013.07.004. Epub 2013 Jul 20. Reprod Biol. 2013. PMID: 24011188 Review.
-
Effects of in vivo prematuration and in vivo final maturation on developmental capacity and quality of pre-implantation embryos.Theriogenology. 2002 Jan 1;57(1):5-20. doi: 10.1016/s0093-691x(01)00655-0. Theriogenology. 2002. PMID: 11775980 Review.
Cited by
-
Advances in Dairy Cattle Reproduction-A Foreword.Animals (Basel). 2024 Sep 12;14(18):2650. doi: 10.3390/ani14182650. Animals (Basel). 2024. PMID: 39335240 Free PMC article.
References
LinkOut - more resources
Full Text Sources

