Mitochondrial Volume Regulation and Swelling Mechanisms in Cardiomyocytes
- PMID: 37627512
- PMCID: PMC10451443
- DOI: 10.3390/antiox12081517
Mitochondrial Volume Regulation and Swelling Mechanisms in Cardiomyocytes
Abstract
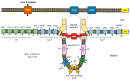
Mitochondrion, known as the "powerhouse" of the cell, regulates ion homeostasis, redox state, cell proliferation and differentiation, and lipid synthesis. The inner mitochondrial membrane (IMM) controls mitochondrial metabolism and function. It possesses high levels of proteins that account for ~70% of the membrane mass and are involved in the electron transport chain, oxidative phosphorylation, energy transfer, and ion transport, among others. The mitochondrial matrix volume plays a crucial role in IMM remodeling. Several ion transport mechanisms, particularly K+ and Ca2+, regulate matrix volume. Small increases in matrix volume through IMM alterations can activate mitochondrial respiration, whereas excessive swelling can impair the IMM topology and initiates mitochondria-mediated cell death. The opening of mitochondrial permeability transition pores, the well-characterized phenomenon with unknown molecular identity, in low- and high-conductance modes are involved in physiological and pathological increases of matrix volume. Despite extensive studies, the precise mechanisms underlying changes in matrix volume and IMM structural remodeling in response to energy and oxidative stressors remain unknown. This review summarizes and discusses previous studies on the mechanisms involved in regulating mitochondrial matrix volume, IMM remodeling, and the crosstalk between these processes.
Keywords: heart; ions; ischemia-reperfusion; mitochondria; permeability transition pore.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Varikmaa M., Guzun R., Grichine A., Gonzalez-Granillo M., Usson Y., Boucher F., Kaambre T., Saks V. Matters of the heart in bioenergetics: Mitochondrial fusion into continuous reticulum is not needed for maximal respiratory activity. J. Bioenerg. Biomembr. 2013;45:319–331. doi: 10.1007/s10863-012-9494-4. - DOI - PubMed
-
- Murphy E., Ardehali H., Balaban R.S., DiLisa F., Dorn G.W., 2nd, Kitsis R.N., Otsu K., Ping P., Rizzuto R., Sack M.N., et al. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association. Circ. Res. 2016;118:1960–1991. doi: 10.1161/RES.0000000000000104. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

