Evaluating the Antioxidant Properties of the Ancient-Crop Tef (Eragrostis tef) Grain Extracts in THP-1 Monocytes
- PMID: 37627557
- PMCID: PMC10451390
- DOI: 10.3390/antiox12081561
Evaluating the Antioxidant Properties of the Ancient-Crop Tef (Eragrostis tef) Grain Extracts in THP-1 Monocytes
Abstract
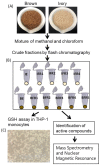
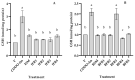
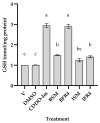
Tef (Eragrostis tef) is an orphan crop that is widely grown in East Africa, primarily in Ethiopia as a staple crop. It is becoming popular in the Western world owing to its nutritious and gluten-free grains and the forage quality of its biomass. Tef is also considered to have a high antioxidant capacity based on cell-free studies. However, the antioxidant activity of tef has never been validated using a physiologically relevant cell model. The purpose of this study was to investigate the antioxidant capacity of tef grain extracts using a mammalian cell model. We hypothesized that the tef grain extracts are capable of modulating the cellular antioxidant response via the modulation of glutathione (GSH) biosynthetic pathways. Therefore, we evaluated the antioxidant activity of purified tef grain extracts in the human acute monocytic leukemia (THP-1) cell line. Our findings revealed that the organic fraction of grain extracts increased the cellular GSH level, which was more evident for brown-colored tef than the ivory variety. Moreover, a brown-tef fraction increased the expressions of GSH-pathway genes, including γ-glutamate cysteine ligase catalytic (GCLC) and modifier (GCLM) subunits and glutathione reductase (GR), an enzyme that plays a key role in GSH biosynthesis, suggesting that tef extracts may modulate GSH metabolism. Several compounds were uniquely identified via mass spectrometry (MS) in GSH-modulating brown-tef samples, including 4-oxo-β-apo-13-carotenone, γ-linolenic acid (methyl ester), 4,4'-(2,3-dimethyl-1,4-butanediyl)bis-phenol (also referred to as 8,8'-lignan-4,4'-diol), and (3β)-3-[[2-[4-(Acetylamino)phenoxy]acetyl]oxy]olean-12-en-28-oic acid. Tef possesses antioxidant activity due to the presence of phytochemicals that can act as direct antioxidants, as well as modulators of antioxidant-response genes, indicating its potential role in alleviating diseases triggered by oxidative stresses. To the best of our knowledge, this is the first report revealing the antioxidant ability of tef extracts in a physiologically relevant human cell model.
Keywords: Eragrostis tef; antioxidant activity; glutathione-pathway genes; grain extracts; phytochemicals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Transcriptomic Profile of Tef (Eragrostis tef) in Response to Drought.Plants (Basel). 2024 Nov 2;13(21):3086. doi: 10.3390/plants13213086. Plants (Basel). 2024. PMID: 39520004 Free PMC article.
-
Seed priming with gas plasma-activated water in Ethiopia's "orphan" crop tef (Eragrostis tef).Planta. 2024 Feb 26;259(4):75. doi: 10.1007/s00425-024-04359-5. Planta. 2024. PMID: 38409565 Free PMC article.
-
Significance and prospects of an orphan crop tef.Planta. 2019 Sep;250(3):753-767. doi: 10.1007/s00425-019-03209-z. Epub 2019 Jun 20. Planta. 2019. PMID: 31222492 Review.
-
Silicon Enhances Biomass and Grain Yield in an Ancient Crop Tef [Eragrostis tef (Zucc.) Trotter].Front Plant Sci. 2020 Nov 26;11:608503. doi: 10.3389/fpls.2020.608503. eCollection 2020. Front Plant Sci. 2020. PMID: 33329679 Free PMC article.
-
Chemical composition and food uses of teff (Eragrostis tef).Food Chem. 2018 Jan 15;239:402-415. doi: 10.1016/j.foodchem.2017.06.101. Epub 2017 Jun 20. Food Chem. 2018. PMID: 28873585 Review.
Cited by
-
Population genomics uncovers loci for trait improvement in the indigenous African cereal tef (Eragrostis tef).Commun Biol. 2025 May 26;8(1):807. doi: 10.1038/s42003-025-08206-5. Commun Biol. 2025. PMID: 40419766 Free PMC article.
-
Biggest of tinies: natural variation in seed size and mineral distribution in the ancient crop tef [Eragrostis tef (Zucc.) Trotter].Front Plant Sci. 2024 Dec 12;15:1485819. doi: 10.3389/fpls.2024.1485819. eCollection 2024. Front Plant Sci. 2024. PMID: 39726428 Free PMC article.
-
Mixed teff (Eragrostis tef, Poaceae) cultivation and consumption among smallholder farmers in South Wollo Zone, Ethiopia.J Ethnobiol Ethnomed. 2025 Apr 24;21(1):27. doi: 10.1186/s13002-025-00776-2. J Ethnobiol Ethnomed. 2025. PMID: 40275288 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

