Synergistic Protective Effect of Fermented Schizandrae Fructus Pomace and Hoveniae Semen cum Fructus Extracts Mixture in the Ethanol-Induced Hepatotoxicity
- PMID: 37627597
- PMCID: PMC10451898
- DOI: 10.3390/antiox12081602
Synergistic Protective Effect of Fermented Schizandrae Fructus Pomace and Hoveniae Semen cum Fructus Extracts Mixture in the Ethanol-Induced Hepatotoxicity
Abstract
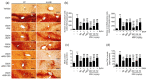
Schizandrae Fructus (SF), fruits of Schisandra chinensis (Turcz.) Baill. and Hoveniae Semen cum Fructus (HSCF), the dried peduncle of Hovenia dulcis Thunb., have long been used for alcohol detoxification in the traditional medicine of Korea and China. In the current study, we aimed to evaluate the potential synergistic hepatoprotective effect of a combination mixture (MSH) comprising fermented SF pomace (fSFP) and HSCF hot water extracts at a 1:1 (w:w) ratio against ethanol-induced liver toxicity. Subacute ethanol-mediated hepatotoxicity was induced by the oral administration of ethanol (5 g/kg) in C57BL/6J mice once daily for 14 consecutive days. One hour after each ethanol administration, MSH (50, 100, and 200 mg/kg) was also orally administered daily. MSH administration significantly reduced the serum activities of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and γ-glutamyl transpeptidase. Histological observation indicated that MSH administration synergistically and significantly decreased the fatty changed region of hepatic parenchyma and the formation of lipid droplet in hepatocytes. Moreover, MSH significantly attenuated the hepatic triglyceride accumulation through reducing lipogenesis genes expression and increasing fatty acid oxidation genes expression. In addition, MSH significantly inhibited protein nitrosylation and lipid peroxidation by lowering cytochrome P450 2E1 enzyme activity and restoring the glutathione level, superoxide dismutase and catalase activity in liver. Furthermore, MSH synergistically decreased the mRNA level of tumor necrosis factor-α in the hepatic tissue. These findings indicate that MSH has potential for preventing alcoholic liver disease through inhibiting hepatic steatosis, oxidative stress, and inflammation.
Keywords: Hoveniae Semen cum Fructus; Schizandrae Fructus; alcoholic liver disease; anti-steatosis; antioxidant; ethanol.
Conflict of interest statement
B.-R.C. and H.-R.P. are employed by Nutracore; however, in this research, they were only involved in the preparation and analysis of the raw materials to a limited extent. K.-H.J., J.-K.K., and S.-K.K. declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

