Peroxiredoxins Play an Important Role in the Regulation of Immunity and Aging in Drosophila
- PMID: 37627611
- PMCID: PMC10451867
- DOI: 10.3390/antiox12081616
Peroxiredoxins Play an Important Role in the Regulation of Immunity and Aging in Drosophila
Abstract
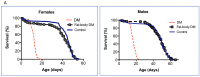
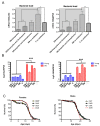
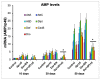
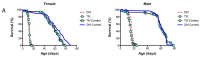
Aberrant immune responses and chronic inflammation can impose significant health risks and promote premature aging. Pro-inflammatory responses are largely mediated via reactive oxygen species (ROS) and reduction-oxidation reactions. A pivotal role in maintaining cellular redox homeostasis and the proper control of redox-sensitive signaling belongs to a family of antioxidant and redox-regulating thiol-related peroxidases designated as peroxiredoxins (Prx). Our recent studies in Drosophila have shown that Prxs play a critical role in aging and immunity. We identified two important 'hubs', the endoplasmic reticulum (ER) and mitochondria, where extracellular and intracellular stress signals are transformed into pro-inflammatory responses that are modulated by the activity of the Prxs residing in these cellular organelles. Here, we found that mitochondrial Prx activity in the intestinal epithelium is required to prevent the development of intestinal barrier dysfunction, which can drive systemic inflammation and premature aging. Using a redox-negative mutant, we demonstrated that Prx acts in a redox-dependent manner in regulating the age-related immune response. The hyperactive immune response observed in flies under-expressing mitochondrial Prxs is due to a response to abiotic signals but not to changes in the bacterial content. This hyperactive response, but not reduced lifespan phenotype, can be rescued by the ER-localized Prx.
Keywords: Drosophila; aging; endoplasmic reticulum; immunity; mitochondria; peroxiredoxin; reactive oxygen species; redox state.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as potential conflict of interest.
Figures




Similar articles
-
Mitochondrial Redox Signaling Is Critical to the Normal Functioning of the Neuronal System.Front Cell Dev Biol. 2021 Jan 28;9:613036. doi: 10.3389/fcell.2021.613036. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33585478 Free PMC article.
-
Mitochondrial peroxiredoxins are essential in regulating the relationship between Drosophila immunity and aging.Biochim Biophys Acta Mol Basis Dis. 2017 Jan;1863(1):68-80. doi: 10.1016/j.bbadis.2016.10.017. Epub 2016 Oct 19. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27770625 Free PMC article.
-
The role of peroxiredoxin 4 in inflammatory response and aging.Biochim Biophys Acta. 2016 Feb;1862(2):265-73. doi: 10.1016/j.bbadis.2015.12.008. Epub 2015 Dec 9. Biochim Biophys Acta. 2016. PMID: 26689888 Free PMC article.
-
Plant thiol peroxidases as redox sensors and signal transducers in abiotic stress acclimation.Free Radic Biol Med. 2022 Nov 20;193(Pt 2):764-778. doi: 10.1016/j.freeradbiomed.2022.11.019. Epub 2022 Nov 17. Free Radic Biol Med. 2022. PMID: 36403735 Review.
-
The Multifaceted Impact of Peroxiredoxins on Aging and Disease.Antioxid Redox Signal. 2018 Nov 1;29(13):1293-1311. doi: 10.1089/ars.2017.7452. Epub 2018 Jan 17. Antioxid Redox Signal. 2018. PMID: 29212351 Free PMC article. Review.
Cited by
-
Insect Peroxiredoxins: A Comprehensive Review of Their Classification, Distribution, Structural Features, Expression Profiles and Physiological Functions.Insects. 2025 Jun 28;16(7):678. doi: 10.3390/insects16070678. Insects. 2025. PMID: 40725309 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

