Melatonin as a Therapy for Preterm Brain Injury: What Is the Evidence?
- PMID: 37627625
- PMCID: PMC10451719
- DOI: 10.3390/antiox12081630
Melatonin as a Therapy for Preterm Brain Injury: What Is the Evidence?
Abstract
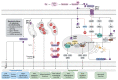
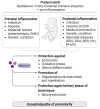
Despite significant improvements in survival following preterm birth in recent years, the neurodevelopmental burden of prematurity, with its long-term cognitive and behavioral consequences, remains a significant challenge in neonatology. Neuroprotective treatment options to improve neurodevelopmental outcomes in preterm infants are therefore urgently needed. Alleviating inflammatory and oxidative stress (OS), melatonin might modify important triggers of preterm brain injury, a complex combination of destructive and developmental abnormalities termed encephalopathy of prematurity (EoP). Preliminary data also suggests that melatonin has a direct neurotrophic impact, emphasizing its therapeutic potential with a favorable safety profile in the preterm setting. The current review outlines the most important pathomechanisms underlying preterm brain injury and correlates them with melatonin's neuroprotective potential, while underlining significant pharmacokinetic/pharmacodynamic uncertainties that need to be addressed in future studies.
Keywords: encephalopathy of prematurity; inflammation; melatonin; neuroprotection; oligodendrocyte dysmaturation; oxidative stress; preterm infants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

