Bioactive Naphtho-α-Pyranones from Two Endophytic Fungi of the Genus Polyphilus
- PMID: 37627693
- PMCID: PMC10451773
- DOI: 10.3390/antibiotics12081273
Bioactive Naphtho-α-Pyranones from Two Endophytic Fungi of the Genus Polyphilus
Abstract
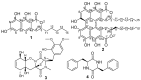
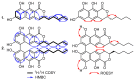
In the course of our survey to study the metabolic potential of two species of a new helotialean genus Polyphilus, namely P. frankenii and P. sieberi, their crude extracts were obtained using different cultivation techniques, which led to the isolation and characterization of two new naphtho-α-pyranone derivatives recognized as a monomer (1) and its 6,6'-homodimer (2) together with two known diketopiperazine congeners, outovirin B (3) and (3S,6S)-3,6-dibenzylpiperazine-2,5-dione (4). The structures of isolated compounds were determined based on extensive 1D and 2D NMR and HRESIMS. The absolute configuration of new naphtho-α-pyranones was determined using a comparison of their experimental ECD spectra with those of related structural analogues. 6,6'-binaphtho-α-pyranone talaroderxine C (2) exhibited potent cytotoxic activity against different mammalian cell lines with IC50 values in the low micromolar to nanomolar range. In addition, talaroderxine C unveiled stronger antimicrobial activity against Bacillus subtilis rather than Staphylococcus aureus with MIC values of 0.52 µg mL-1 (0.83 µM) compared to 66.6 µg mL-1 (105.70 µM), respectively.
Keywords: Ascomycota; Helotiales; Polyphilus; antimicrobial; naphthopyranones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ayer: W.A., Craw P.A., Nozawa K. Two 1H-naphtho [2,3-c]pyran-1-one metabolites from the fungus Paecilomyces variotii. Can. J. Chem. 1991;69:189–191. doi: 10.1139/v91-030. - DOI
-
- Liu J., Li F., Kim E.L., Hong J., Jung H.J. Viriditoxin, from a jellyfish-derived fungus, is antibiotic to fish pathogens. Nat. Prod. Sci. 2013;19:61–65.
-
- Elix J.A., Wardlaw J.H. Pigmentosin A, a new naphthopyrone from the lichen Hyptotrachyna immaculata. Aust. J. Chem. 2004;57:681–683. doi: 10.1071/CH04003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases