Update on the Pathogenesis of Enteropathy-Associated T-Cell Lymphoma
- PMID: 37627888
- PMCID: PMC10453492
- DOI: 10.3390/diagnostics13162629
Update on the Pathogenesis of Enteropathy-Associated T-Cell Lymphoma
Abstract
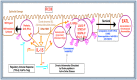
EATL is an aggressive T-cell non-Hodgkin lymphoma with poor prognosis and is largely localized to the small intestine. EATL is closely associated with coeliac disease (CD) and is seen mostly in patients originating from Northern Europe. Various factors are associated with an increased risk of developing EATL, such as viral infection, advanced age, being male, and the presence of the HLA-DQ2 haplotype. Clonal rearrangements in the TCR-β and γ genes have been reported in all EATL morphological variants with distinctive immunophenotypic characteristics. Although EATL can occur de novo, individuals with RCDII are at a higher risk of developing EATL. The cells of origin of EATL has been postulated to be normal small intestinal intraepithelial T-lymphocytes (IELs), and more recent evidence suggests a link between innate precursor IELs and EATL derived from refractory coeliac disease type II (RCDII). The immune microenvironment of mucosal cells within the small intestine enhances the process of neoplastic transformation of IELs into EATL. Cytokines such as IL-15 can activate and crucially deregulate the JAK-STAT signaling pathway by binding to receptors on the surface of IELs. Furthermore, mutations in the JAK/STAT pathway have been associated with RCDII-derived EATL.
Keywords: EATL; T-cell non-Hodgkin lymphoma; coeliac disease; genetics; intraepithelial T-lymphocytes; refractory coeliac disease; small intestine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fei F., Reddy V., Patel C.R., Dhall D., Lee G., Meng-Jun X., Al Diffalha S. Monomorphic Epitheliotropic Intestinal T-cell Lymphoma: A Study of Four Cases and Review of Literature. Ann. Clin. Lab. Sci. 2020;50:806–812. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

