Investigation of Cognitive Impairment in the Course of Post-COVID Syndrome
- PMID: 37627961
- PMCID: PMC10453167
- DOI: 10.3390/diagnostics13162703
Investigation of Cognitive Impairment in the Course of Post-COVID Syndrome
Abstract
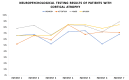
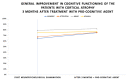
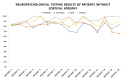
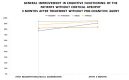
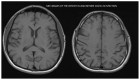
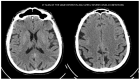
(1) Background: The study presents results from an investigation of cognitive impairment in patients hospitalized in the first psychiatric clinic in Bulgaria to treat patients with COVID-19 during the pandemic period between 2020 and 2022. One hundred and twenty patients who had recovered from acute COVID-19 infection (up to 12 weeks ago) and had no previous history of cognitive impairment participated in the study. In 23 of them (19.17%), disturbance of cognitive functioning was observed. (2) Methods: All 23 patients underwent neuropsychological (Luria's test, Platonov's Maze test, MMSE, Boston Naming test) and neuroimaging examinations. Only seven of them had evidence of cortical atrophy on CT/MRI images. The most significantly demonstrative image of one of those patients is presented. (3) Results: The neuropsychological testing results of both groups show a certain decrease in fixation and memory retention as well as in the range, concentration, distribution and switching of attention. Deviations from the norm on the MMSE, as well as on the Boston Naming Test, were found in the group of patients with cortical atrophy (mild to moderate aphasia). Neuroprotective agents such as Citicoline, Piracetam and Memantine were prescribed to the patients with evident cortical atrophy. After 3 months, positive results of the neuropsychological examination were reported in both groups. (4) Conclusions: Although there are limited data on the benefit of prescribing pro-cognitive agents in the post-COVID period, our clinical experience suggests that it might be useful in the recovery process from the infection's consequences on cognition for patients with brain pathology.
Keywords: COVID-19; aphasia; attention deficit; cognitive disturbance; cognitive impairment; memory; mild cognitive deficit; neuroprotective agent; post-COVID syndrome; pro-cognitive agent.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[Clinical presentation of Moroccan cases with Alzheimer's disease].Encephale. 2014 Dec;40(6):481-6. doi: 10.1016/j.encep.2014.06.006. Epub 2014 Aug 12. Encephale. 2014. PMID: 25127896 French.
-
American Medical Society for Sports Medicine position statement: concussion in sport.Br J Sports Med. 2013 Jan;47(1):15-26. doi: 10.1136/bjsports-2012-091941. Br J Sports Med. 2013. PMID: 23243113 Review.
-
Functional connectivity underlying cognitive and psychiatric symptoms in post-COVID-19 syndrome: is anosognosia a key determinant?Brain Commun. 2022 Mar 9;4(2):fcac057. doi: 10.1093/braincomms/fcac057. eCollection 2022. Brain Commun. 2022. PMID: 35350554 Free PMC article.
-
[Correlation of muscle strength with cognitive function and medial temporal lobe atrophy in patients with mild to moderate Alzheimer's disease].Zhonghua Yi Xue Za Zhi. 2022 Sep 20;102(35):2786-2792. doi: 10.3760/cma.j.cn112137-20220406-00715. Zhonghua Yi Xue Za Zhi. 2022. PMID: 36124351 Chinese.
-
"Brain-specific" nutrients: a memory cure?Nutrition. 2003 Nov-Dec;19(11-12):957-75. doi: 10.1016/s0899-9007(03)00024-8. Nutrition. 2003. PMID: 14624946 Review.
References
-
- Hellmuth J., Barnett T.A., Asken B.M., Kelly J.D., Torres L., Stephens M.L., Greenhouse B., Martin J.N., Chow F.C., Deeks S.G., et al. Persistent COVID-19-associated neurocognitive symptoms in non-hospitalized patients. J. Neurovirology. 2021;27:191–195. doi: 10.1007/s13365-021-00954-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

