Improving the Thermostability of Serine Protease PB92 from Bacillus alcalophilus via Site-Directed Mutagenesis Based on Semi-Rational Design
- PMID: 37628080
- PMCID: PMC10453622
- DOI: 10.3390/foods12163081
Improving the Thermostability of Serine Protease PB92 from Bacillus alcalophilus via Site-Directed Mutagenesis Based on Semi-Rational Design
Abstract
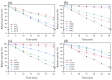
Proteases have been widely employed in many industrial processes. In this work, we aimed to improve the thermostability of the serine protease PB92 from Bacillus alcalophilus to meet the high-temperature requirements of biotechnological treatments. Eight mutation sites (N18, S97-S101, E110, and R143) were identified, and 21 mutants were constructed from B-factor comparison and multiple sequence alignment and expressed via Bacillus subtilis. Among them, fifteen mutants exhibited increased half-life (t1/2) values at 65 °C (1.13-31.61 times greater than that of the wild type). Based on the composite score of enzyme activity and thermostability, six complex mutants were implemented. The t1/2 values of these six complex mutants were 2.12-10.05 times greater than that of the wild type at 65 °C. In addition, structural analysis revealed that the increased thermal stability of complex mutants may be related to the formation of additional hydrophobic interactions due to increased hydrophobicity and the decreased flexibility of the structure. In brief, the thermal stability of the complex mutants N18L/R143L/S97A, N18L/R143L/S99L, and N18L/R143L/G100A was increased 4-fold, which reveals application potential in industry.
Keywords: B-factor; protease; site-directed mutagenesis; thermostability; weighted analysis.
Conflict of interest statement
The authors have no financial or other conflicts of interest to declare.
Figures




References
-
- Martin J.R., Mulder F.A., Karimi-Nejad Y., van der Zwan J., Mariani M., Schipper D., Boelens R. The solution structure of serine protease PB92 from Bacillus alcalophilus presents a rigid fold with a flexible substrate-binding site. Structure. 1997;5:521–532. doi: 10.1016/S0969-2126(97)00208-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources

