The Protective Effect and Mechanism of a Phytochemical Extract from the Wild Vegetable Shutou (Crateva unilocularis Buch.) against Acetaminophen-Induced Liver Injury in Mice
- PMID: 37628108
- PMCID: PMC10453156
- DOI: 10.3390/foods12163109
The Protective Effect and Mechanism of a Phytochemical Extract from the Wild Vegetable Shutou (Crateva unilocularis Buch.) against Acetaminophen-Induced Liver Injury in Mice
Abstract
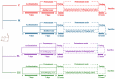
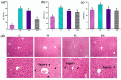
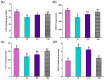
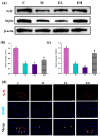
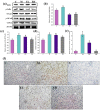
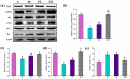
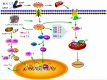
Acetaminophen (APAP) abuse is a common public health problem which can cause severe liver damage. However, strategies for dealing with this situation safely and effectively are very limited. The goal of the current work was to evaluate the protection and potential molecular mechanisms of an ethanol extract from shoots of the wild vegetable shutou (Crateva unilocularis Buch.) (ECS) against APAP-induced liver damage in mice. Mice orally received ECS for seven days (300 or 600 mg/kg b.w. per day) before being intraperitoneally injected with APAP (250 mg/kg). Results exhibited that ECS obviously decreased the content of alkaline phosphatase, alanine aminotransferase, aspartate transaminase, and malondialdehyde (p < 0.05). Catalase and superoxide dismutase were notably restored (p < 0.05), and the content of reduced glutathione was obviously increased (p < 0.05). Moreover, ECS significantly inhibited the secretion of interleukin-1β and tumor necrosis factor-α (p < 0.05). Further analyses of the mechanisms showed that ECS may alleviate oxidative stress in the liver by increasing the expression of the nuclear factor erythroid-2-related factor 2 and NADH quinone oxidoreductase 1 proteins, and may suppress liver inflammation by inhibiting the expression of the phosphorylated-inhibitor kappa B alpha/inhibitor kappa B alpha, phosphorylated-nuclear factor κB/nuclear factor κB, and cyclooxygenase-2 proteins. Meanwhile, ECS inhibited hepatocyte apoptosis by enhancing B-cell lymphoma gene 2 and suppressing Bcl-2-associated X protein. In summary, ECS may be used as a dietary supplement to prevent the liver damage caused by APAP abuse.
Keywords: APAP; Crateva unilocularis Buch.; liver damage; reactive oxygen species.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
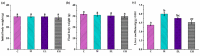

Similar articles
-
Improved protective effects of American ginseng berry against acetaminophen-induced liver toxicity through TNF-α-mediated caspase-3/-8/-9 signaling pathways.Phytomedicine. 2018 Dec 1;51:128-138. doi: 10.1016/j.phymed.2018.09.234. Epub 2018 Oct 2. Phytomedicine. 2018. PMID: 30466610
-
Protective Effects of Tormentic Acid, a Major Component of Suspension Cultures of Eriobotrya japonica Cells, on Acetaminophen-Induced Hepatotoxicity in Mice.Molecules. 2017 May 18;22(5):830. doi: 10.3390/molecules22050830. Molecules. 2017. PMID: 28524081 Free PMC article.
-
The protective efficacy of soursop fruit extract against hepatic injury associated with acetaminophen exposure is mediated through antioxidant, anti-inflammatory, and anti-apoptotic activities.Environ Sci Pollut Res Int. 2019 May;26(13):13539-13550. doi: 10.1007/s11356-019-04935-3. Epub 2019 Mar 26. Environ Sci Pollut Res Int. 2019. PMID: 30915694
-
Saponins (Ginsenosides) from the Leaves of Panax quinquefolius Ameliorated Acetaminophen-Induced Hepatotoxicity in Mice.J Agric Food Chem. 2017 May 10;65(18):3684-3692. doi: 10.1021/acs.jafc.7b00610. Epub 2017 Apr 25. J Agric Food Chem. 2017. PMID: 28429935
-
Protective effects of α-mangostin against acetaminophen-induced acute liver injury in mice.Eur J Pharmacol. 2018 May 15;827:173-180. doi: 10.1016/j.ejphar.2018.03.002. Epub 2018 Mar 18. Eur J Pharmacol. 2018. PMID: 29563064
References
-
- Wang Y., Tian L., Wang Y., Zhao T., Khan A., Wang Y., Cao J., Cheng G. Protective effect of Que Zui tea hot-water and aqueous ethanol extract against acetaminophen-induced liver injury in mice via inhibition of oxidative stress, inflammation, and apoptosis. Food Funct. 2021;12:2468–2480. doi: 10.1039/D0FO02894K. - DOI - PubMed
-
- Sun Y., Ma N., Liu X., Yi J., Cai S. Preventive effects of Chinese sumac fruits against acetaminophen-induced liver injury in mice via regulating oxidative stress, inflammation and apoptosis. J. Funct. Foods. 2021;87:104830. doi: 10.1016/j.jff.2021.104830. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

