Advances in Genetic Tools and Their Application in Streptococcus thermophilus
- PMID: 37628118
- PMCID: PMC10453384
- DOI: 10.3390/foods12163119
Advances in Genetic Tools and Their Application in Streptococcus thermophilus
Abstract
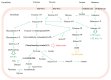
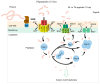
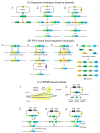
Streptococcus thermophilus is a traditional starter. Nowadays, key aspects of S. thermophilus physiology have been revealed concerning the phenotypic traits relevant for industrial applications, including sugar metabolism, protein hydrolysis, and the production of important metabolites that affect the sensory properties of fermented foods as well as the original cooperation with Lactobacillus delbrueckii subsp. bulgaricus. Moreover, significant advances have been made in the synthetic biology toolbox of S. thermophilus based on technological advances in the genome and its sequencing and synthesis. In this review, we discuss the recently developed toolbox for S. thermophilus, including gene expression toolsets (promoters, terminators, plasmids, etc.) and genome editing tools. It can be used for both functionalized foods and therapeutic molecules for consumers. The availability of new molecular tools, including the genome editing toolbox, has facilitated the engineering of physiological studies of S. thermophilus and the generation of strains with improved technical and functional characteristics.
Keywords: Streptococcus thermophilus; genetic tools; plasmid; production characteristics; promoters.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships.
Figures
Similar articles
-
Harnessing the metabolic potential of Streptococcus thermophilus for new biotechnological applications.Curr Opin Biotechnol. 2020 Feb;61:142-152. doi: 10.1016/j.copbio.2019.12.019. Epub 2020 Jan 13. Curr Opin Biotechnol. 2020. PMID: 31945498 Review.
-
Isolation and characterization of yogurt starter cultures from traditional yogurts and growth kinetics of selected cultures under lab-scale fermentation.Prep Biochem Biotechnol. 2023;53(4):454-463. doi: 10.1080/10826068.2022.2098325. Epub 2022 Jul 18. Prep Biochem Biotechnol. 2023. PMID: 35848985
-
Effect of lactose hydrolysis on the milk-fermenting properties of Lactobacillus delbrueckii ssp. bulgaricus 2038 and Streptococcus thermophilus 1131.J Dairy Sci. 2021 Feb;104(2):1454-1464. doi: 10.3168/jds.2020-19244. Epub 2020 Dec 11. J Dairy Sci. 2021. PMID: 33309355
-
Enhancing the Sweetness of Yoghurt through Metabolic Remodeling of Carbohydrate Metabolism in Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus.Appl Environ Microbiol. 2016 May 31;82(12):3683-3692. doi: 10.1128/AEM.00462-16. Print 2016 Jun 15. Appl Environ Microbiol. 2016. PMID: 27107115 Free PMC article.
-
New Insights into Various Production Characteristics of Streptococcus thermophilus Strains.Int J Mol Sci. 2016 Oct 12;17(10):1701. doi: 10.3390/ijms17101701. Int J Mol Sci. 2016. PMID: 27754312 Free PMC article. Review.
Cited by
-
Fruit and Vegetable Juices as Functional Carriers for Probiotic Delivery: Microbiological, Nutritional, and Sensory Perspectives.Microorganisms. 2025 May 30;13(6):1272. doi: 10.3390/microorganisms13061272. Microorganisms. 2025. PMID: 40572160 Free PMC article. Review.
References
-
- Iyer R., Tomar S.K., Maheswari T., Singh R. Streptococcus thermophilus strains: Multifunctional lactic acid bacteria. Int. Dairy J. 2010;20:133–141. doi: 10.1016/j.idairyj.2009.10.005. - DOI
-
- Alexandraki V., Kazou M., Blom J., Pot B., Papadimitriou K., Tsakalidou E. Comparative Genomics of Streptococcus thermophilus Support Important Traits Concerning the Evolution, Biology and Technological Properties of the Species. Front. Microbiol. 2019;10:2916. doi: 10.3389/fmicb.2019.02916. - DOI - PMC - PubMed
-
- Bolotin A., Quinquis B., Renault P., Sorokin A., Ehrlich S.D., Kulakauskas S., Lapidus A., Goltsman E., Mazur M., Pusch G.D., et al. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat. Biotechnol. 2004;22:1554–1558. doi: 10.1038/nbt1034. - DOI - PMC - PubMed
Publication types
Grants and funding
- Grant No. 2020CXGC010602/the Key Research and Development Program of Shandong Province
- Grant No. 2020KJE005/he Science and Technology Support Plan for Young People in Colleges and Universities of Shandong Province
- Grant No. ZR2022MC059/Natural Science oundation of Shandong Province
- Grant No. 2022JBZ01-06/Key innovation Project of Qilu University of Technology (Shandong Academy of Sciences)
LinkOut - more resources
Full Text Sources

