The Effectiveness of Home-Based Inspiratory Muscle Training on Small Airway Function and Disease-Associated Symptoms in Patients with Chronic Obstructive Pulmonary Disease
- PMID: 37628507
- PMCID: PMC10454373
- DOI: 10.3390/healthcare11162310
The Effectiveness of Home-Based Inspiratory Muscle Training on Small Airway Function and Disease-Associated Symptoms in Patients with Chronic Obstructive Pulmonary Disease
Abstract
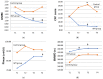
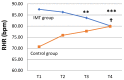
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitations, occurring mainly in the small airways. Weakness in the respiratory muscles contributes to dyspnea and a decreased exercise capacity in COPD patients. This study aimed to investigate the effectiveness of home-based inspiratory muscle training (IMT) on small airway function and symptoms in COPD patients. This research adopted a non-randomized controlled-study quasi-experimental design. The IMT program consisted of two 15 min sessions·d-1, 5 d·wk-1, with 40% of the maximal inspiratory pressure (PImax) on each participant's assessment results and lasted for 12 weeks. Small airway function was assessed using plethysmography at baseline and after 12 weeks. The modified British Medical Research Council (mMRC), COPD assessment test (CAT), PImax, and 6 min walking distance (6MWD) were recorded at baseline as well as four, eight, and twelve weeks. Twenty-three participants with at least moderate COPD were enrolled in IMT (n = 16) or in the control group (n = 7) in this study. The study participants were mostly male (82.6%), and the average age was 68.29 ± 10.87 years, with a mean body mass index (BMI) of 23.54 ± 4.79. After 12 weeks, the ratios of the first second of forced expiration to the forced vital capacity (FEV1/FVC%) (B coefficient [95% Wald confidence interval] of 5.21 [0.46 to 9.96], p = 0.032), forced expiratory flow (FEF25-75%) (0.20 [0.04 to 0.35] L/s, p = 0.012), and FEF50% (0.26 [0.08 to 0.43] L/s, p = 0.004) in the IMT group were significantly better than in the control group. The IMT group showed significantly lower CAT scores at week 8 (-5.50 [-10.31 to -0.695] scores, p = 0.025) than the control group. The mMRC grade, CAT score, PImax, and 6MWD were significantly improved compared to their values at baseline in the IMT group. Home-based IMT effectively improved post-bronchodilator small airway function and disease-associated symptoms in COPD patients.
Keywords: chronic obstructive pulmonary disease; dyspnea; home-based pulmonary rehabilitation; inspiratory muscle training; small airway function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Evaluation of the effectiveness of a home-based inspiratory muscle training programme in patients with chronic obstructive pulmonary disease using multiple inspiratory muscle tests.Disabil Rehabil. 2016;38(3):250-9. doi: 10.3109/09638288.2015.1036171. Epub 2015 Apr 17. Disabil Rehabil. 2016. PMID: 25885668 Clinical Trial.
-
Inspiratory muscle training reduces diaphragm activation and dyspnea during exercise in COPD.J Appl Physiol (1985). 2018 Aug 1;125(2):381-392. doi: 10.1152/japplphysiol.01078.2017. Epub 2018 Mar 15. J Appl Physiol (1985). 2018. PMID: 29543134 Clinical Trial.
-
Inspiratory muscle training during pulmonary rehabilitation in chronic obstructive pulmonary disease: A randomized trial.Chron Respir Dis. 2015 Nov;12(4):305-12. doi: 10.1177/1479972315594625. Epub 2015 Jul 13. Chron Respir Dis. 2015. PMID: 26170421 Clinical Trial.
-
Effectiveness and safety of inspiratory muscle training in patients with pulmonary hypertension: A systematic review and meta-analysis.Front Cardiovasc Med. 2022 Nov 29;9:999422. doi: 10.3389/fcvm.2022.999422. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36523361 Free PMC article.
-
Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis.Clin Respir J. 2018 Jul;12(7):2178-2188. doi: 10.1111/crj.12905. Epub 2018 May 23. Clin Respir J. 2018. PMID: 29665262
Cited by
-
Effect of Tai Ji and/or Qigong on patients with stable chronic obstructive pulmonary disease: A meta-analysis and systematic review.Medicine (Baltimore). 2025 Jan 31;104(5):e41390. doi: 10.1097/MD.0000000000041390. Medicine (Baltimore). 2025. PMID: 39889182 Free PMC article.
References
-
- Agusti A., Beasley R., Celli B.R., Criner G., Halpin D., Varela M.V., Montes de Oca M., Mortimer K., Salvi S., Vogelmeier C., et al. Pocket Guide to COPD Diagnosis, Management, and Prevention. A Guide for Health Care Professional. Global Initiative for Chronic Obstructive Lung Disease; Deer Park, IL, USA: 2023.
-
- Rabe K.F., Hurd S., Anzueto A., Barnes P.J., Buist S.A., Calverley P., Fukuch Y., Jenkins C., Rodriguez-Roisin R., Well C., et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

