Insights from a Computational-Based Approach for Analyzing Autophagy Genes across Human Cancers
- PMID: 37628602
- PMCID: PMC10454514
- DOI: 10.3390/genes14081550
Insights from a Computational-Based Approach for Analyzing Autophagy Genes across Human Cancers
Abstract
In the last decade, there has been a boost in autophagy reports due to its role in cancer progression and its association with tumor resistance to treatment. Despite this, many questions remain to be elucidated and explored among the different tumors. Here, we used omics-based cancer datasets to identify autophagy genes as prognostic markers in cancer. We then combined these findings with independent studies to further characterize the clinical significance of these genes in cancer. Our observations highlight the importance of innovative approaches to analyze tumor heterogeneity, potentially affecting the expression of autophagy-related genes with either pro-tumoral or anti-tumoral functions. In silico analysis allowed for identifying three genes (TBC1D12, KERA, and TUBA3D) not previously described as associated with autophagy pathways in cancer. While autophagy-related genes were rarely mutated across human cancers, the expression profiles of these genes allowed the clustering of different cancers into three independent groups. We have also analyzed datasets highlighting the effects of drugs or regulatory RNAs on autophagy. Altogether, these data provide a comprehensive list of targets to further the understanding of autophagy mechanisms in cancer and investigate possible therapeutic targets.
Keywords: autophagy; cancer; cancer dataset; cell phenotype; systems biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



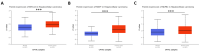
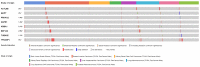

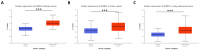
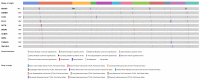
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

