Complement System Activation Is a Plasma Biomarker Signature during Malaria in Pregnancy
- PMID: 37628675
- PMCID: PMC10454407
- DOI: 10.3390/genes14081624
Complement System Activation Is a Plasma Biomarker Signature during Malaria in Pregnancy
Abstract
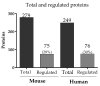
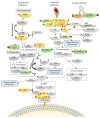
Malaria in pregnancy (MiP) is a public health problem in malaria-endemic areas, contributing to detrimental outcomes for both mother and fetus. Primigravida and second-time mothers are most affected by severe anemia complications and babies with low birth weight compared to multigravida women. Infected erythrocytes (IE) reach the placenta, activating the immune response by placental monocyte infiltration and inflammation. However, specific markers of MiP result in poor outcomes, such as low birth weight, and intrauterine growth restriction for babies and maternal anemia in women infected with Plasmodium falciparum are limited. In this study, we identified the plasma proteome signature of a mouse model infected with Plasmodium berghei ANKA and pregnant women infected with Plasmodium falciparum infection using quantitative mass spectrometry-based proteomics. A total of 279 and 249 proteins were quantified in murine and human plasma samples, of which 28% and 30% were regulated proteins, respectively. Most of the regulated proteins in both organisms are involved in complement system activation during malaria in pregnancy. CBA anaphylatoxin assay confirmed the complement system activation by the increase in C3a and C4a anaphylatoxins in the infected plasma compared to non-infected plasma. Moreover, correlation analysis showed the association between complement system activation and reduced head circumference in newborns from Pf-infected mothers. The data obtained in this study highlight the correlation between the complement system and immune and newborn outcomes resulting from malaria in pregnancy.
Keywords: Plasmodium berghei; Plasmodium falciparum; biomarkers; complement system; malaria; malaria in pregnancy; plasma proteomics.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Complement activation in primiparous women from a malaria endemic area is associated with reduced birthweight.Placenta. 2013 Feb;34(2):162-7. doi: 10.1016/j.placenta.2012.11.030. Epub 2012 Dec 20. Placenta. 2013. PMID: 23261341
-
Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life.Am J Epidemiol. 1997 Nov 15;146(10):826-31. doi: 10.1093/oxfordjournals.aje.a009200. Am J Epidemiol. 1997. PMID: 9384203
-
Pregnancy outcome and placenta pathology in Plasmodium berghei ANKA infected mice reproduce the pathogenesis of severe malaria in pregnant women.PLoS One. 2008 Feb 13;3(2):e1608. doi: 10.1371/journal.pone.0001608. PLoS One. 2008. PMID: 18270595 Free PMC article.
-
Cytoadhesion of Plasmodium falciparum-infected erythrocytes and the infected placenta: a two-way pathway.Braz J Med Biol Res. 2006 Dec;39(12):1525-36. doi: 10.1590/s0100-879x2006001200003. Braz J Med Biol Res. 2006. PMID: 17160261 Review.
-
VAR2CSA-Mediated Host Defense Evasion of Plasmodium falciparum Infected Erythrocytes in Placental Malaria.Front Immunol. 2021 Feb 9;11:624126. doi: 10.3389/fimmu.2020.624126. eCollection 2020. Front Immunol. 2021. PMID: 33633743 Free PMC article. Review.
Cited by
-
Protective Vaccination of Mice Against Blood-Stage Malaria Impacts Hepatic Expression of Genes Encoding Acute-Phase Proteins and IL-6 Family Members.Int J Mol Sci. 2025 Mar 29;26(7):3173. doi: 10.3390/ijms26073173. Int J Mol Sci. 2025. PMID: 40243929 Free PMC article.
-
Plasma protein biomarkers of Plasmodium falciparum infection in pregnant women: a high-throughput proteomics study.Front Cell Infect Microbiol. 2025 Jul 8;15:1594088. doi: 10.3389/fcimb.2025.1594088. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40697816 Free PMC article.
References
-
- World Health Organization . World Malaria Report 2022. World Health Organization; Geneva, Switzerland: 2022.
-
- Ayres Pereira M., Mandel Clausen T., Pehrson C., Mao Y., Resende M., Daugaard M., Riis Kristensen A., Spliid C., Mathiesen L., Knudsen L.E. Placental sequestration of Plasmodium falciparum malaria parasites is mediated by the interaction between VAR2CSA and chondroitin sulfate A on syndecan-1. PLoS Pathog. 2016;12:e1005831. doi: 10.1371/journal.ppat.1005831. - DOI - PMC - PubMed
-
- Stanisic D.I., Moore K.A., Baiwog F., Ura A., Clapham C., King C.L., Siba P.M., Beeson J.G., Mueller I., Fowkes F.J. Risk factors for malaria and adverse birth outcomes in a prospective cohort of pregnant women resident in a high malaria transmission area of Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 2015;109:313–324. doi: 10.1093/trstmh/trv019. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

