SARS-CoV-2 Spike Proteins and Cell-Cell Communication Induce P-Selectin and Markers of Endothelial Injury, NETosis, and Inflammation in Human Lung Microvascular Endothelial Cells and Neutrophils: Implications for the Pathogenesis of COVID-19 Coagulopathy
- PMID: 37628764
- PMCID: PMC10454213
- DOI: 10.3390/ijms241612585
SARS-CoV-2 Spike Proteins and Cell-Cell Communication Induce P-Selectin and Markers of Endothelial Injury, NETosis, and Inflammation in Human Lung Microvascular Endothelial Cells and Neutrophils: Implications for the Pathogenesis of COVID-19 Coagulopathy
Abstract
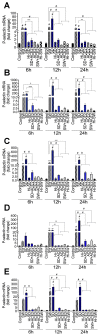
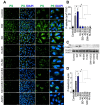
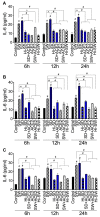
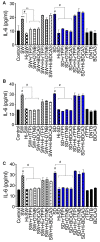
COVID-19 progression often involves severe lung injury, inflammation, coagulopathy, and leukocyte infiltration into pulmonary tissues. The pathogenesis of these complications is unknown. Because vascular endothelium and neutrophils express angiotensin-converting enzyme-2 and spike (S)-proteins, which are present in bodily fluids and tissues of SARS-CoV-2-infected patients, we investigated the effect of S-proteins and cell-cell communication on human lung microvascular endothelial cells and neutrophils expression of P-selectin, markers of coagulopathy, NETosis, and inflammation. Exposure of endothelial cells or neutrophils to S-proteins and endothelial-neutrophils co-culture induced P-selectin transcription and expression, significantly increased expression/secretion of IL-6, von Willebrand factor (vWF, pro-coagulant), and citrullinated histone H3 (cit-H3, NETosis marker). Compared to the SARS-CoV-2 Wuhan variant, Delta variant S-proteins induced 1.4-15-fold higher P-selectin and higher IL-6 and vWF. Recombinant tissue factor pathway inhibitor (rTFPI), 5,5'-dithio-bis-(2-nitrobenzoic acid) (thiol blocker), and thrombomodulin (anticoagulant) blocked S-protein-induced vWF, IL-6, and cit-H3. This suggests that following SARS-CoV-2 contact with the pulmonary endothelium or neutrophils and endothelial-neutrophil interactions, S-proteins increase adhesion molecules, induce endothelial injury, inflammation, NETosis and coagulopathy via the tissue factor pathway, mechanisms involving functional thiol groups, and/or the fibrinolysis system. Using rTFPI, effectors of the fibrinolysis system and/or thiol-based drugs could be viable therapeutic strategies against SARS-CoV-2-induced endothelial injury, inflammation, NETosis, and coagulopathy.
Keywords: DTNB; IL-6; P-selectin; SARS-CoV-2 spike proteins; TFPI; citrullinated histone H3; human lung endothelial cells; neutrophils; neutrophils extracellular traps; thrombomodulin; von willebrand factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
SARS-CoV-2 Spike Proteins and Cell-Cell Communication Inhibits TFPI and Induces Thrombogenic Factors in Human Lung Microvascular Endothelial Cells and Neutrophils: Implications for COVID-19 Coagulopathy Pathogenesis.Int J Mol Sci. 2022 Sep 9;23(18):10436. doi: 10.3390/ijms231810436. Int J Mol Sci. 2022. PMID: 36142345 Free PMC article.
-
Endothelial thrombomodulin downregulation caused by hypoxia contributes to severe infiltration and coagulopathy in COVID-19 patient lungs.EBioMedicine. 2022 Jan;75:103812. doi: 10.1016/j.ebiom.2022.103812. Epub 2022 Jan 13. EBioMedicine. 2022. PMID: 35033854 Free PMC article.
-
SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation.Front Immunol. 2022 Mar 7;13:827146. doi: 10.3389/fimmu.2022.827146. eCollection 2022. Front Immunol. 2022. PMID: 35320941 Free PMC article.
-
Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19).Microvasc Res. 2021 Sep;137:104188. doi: 10.1016/j.mvr.2021.104188. Epub 2021 May 20. Microvasc Res. 2021. PMID: 34022205 Free PMC article. Review.
-
Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes.Int J Mol Sci. 2020 Jul 21;21(14):5168. doi: 10.3390/ijms21145168. Int J Mol Sci. 2020. PMID: 32708334 Free PMC article. Review.
Cited by
-
Impaired instructive and protective barrier functions of the endothelial cell glycocalyx pericellular matrix is impacted in COVID-19 disease.J Cell Mol Med. 2024 Aug;28(16):e70033. doi: 10.1111/jcmm.70033. J Cell Mol Med. 2024. PMID: 39180511 Free PMC article. Review.
-
Mechanisms of lung endothelial cell injury and survival in pulmonary arterial hypertension.Am J Physiol Lung Cell Mol Physiol. 2024 Dec 1;327(6):L972-L983. doi: 10.1152/ajplung.00208.2024. Epub 2024 Oct 15. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39406383 Free PMC article. Review.
-
Recombinant Rod Domain of Vimentin Reduces SARS-CoV-2 Viral Replication by Blocking Spike Protein-ACE2 Interactions.Int J Mol Sci. 2024 Feb 20;25(5):2477. doi: 10.3390/ijms25052477. Int J Mol Sci. 2024. PMID: 38473724 Free PMC article.
-
The Ways of the Virus: Interactions of Platelets and Red Blood Cells with SARS-CoV-2, and Their Potential Pathophysiological Significance in COVID-19.Int J Mol Sci. 2023 Dec 9;24(24):17291. doi: 10.3390/ijms242417291. Int J Mol Sci. 2023. PMID: 38139118 Free PMC article. Review.
-
Short Survey on the Protein Modifications in Plasma during SARS-CoV-2 Infection.Int J Mol Sci. 2023 Sep 14;24(18):14109. doi: 10.3390/ijms241814109. Int J Mol Sci. 2023. PMID: 37762413 Free PMC article.
References
-
- JHU Coronavirus Resource Center: COVID-19 in the USA. 2023. [(accessed on 24 April 2023)]. Available online: https://coronavirus.jhu.edu/
-
- CDC . United States COVID-19 Cases and Deaths by State. US Center for Disease Control and Prevention; Atlanta, GA, USA: 2023. [(accessed on 24 April 2023)]. Available online: https://www.cdc.gov/covid-data-tracker/#cases.
-
- WHO . Coronavirus Disease (COVID-19) Pandemic. World Health Organization; Geneva, Switzerland: 2023. [(accessed on 24 April 2023)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

