SARS-CoV-2 Spike Proteins and Cell-Cell Communication Induce P-Selectin and Markers of Endothelial Injury, NETosis, and Inflammation in Human Lung Microvascular Endothelial Cells and Neutrophils: Implications for the Pathogenesis of COVID-19 Coagulopathy
- PMID: 37628764
- PMCID: PMC10454213
- DOI: 10.3390/ijms241612585
SARS-CoV-2 Spike Proteins and Cell-Cell Communication Induce P-Selectin and Markers of Endothelial Injury, NETosis, and Inflammation in Human Lung Microvascular Endothelial Cells and Neutrophils: Implications for the Pathogenesis of COVID-19 Coagulopathy
Abstract
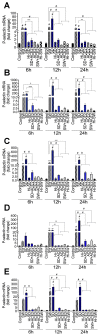
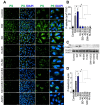
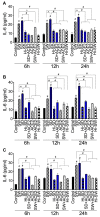
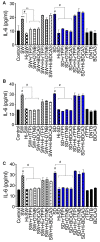
COVID-19 progression often involves severe lung injury, inflammation, coagulopathy, and leukocyte infiltration into pulmonary tissues. The pathogenesis of these complications is unknown. Because vascular endothelium and neutrophils express angiotensin-converting enzyme-2 and spike (S)-proteins, which are present in bodily fluids and tissues of SARS-CoV-2-infected patients, we investigated the effect of S-proteins and cell-cell communication on human lung microvascular endothelial cells and neutrophils expression of P-selectin, markers of coagulopathy, NETosis, and inflammation. Exposure of endothelial cells or neutrophils to S-proteins and endothelial-neutrophils co-culture induced P-selectin transcription and expression, significantly increased expression/secretion of IL-6, von Willebrand factor (vWF, pro-coagulant), and citrullinated histone H3 (cit-H3, NETosis marker). Compared to the SARS-CoV-2 Wuhan variant, Delta variant S-proteins induced 1.4-15-fold higher P-selectin and higher IL-6 and vWF. Recombinant tissue factor pathway inhibitor (rTFPI), 5,5'-dithio-bis-(2-nitrobenzoic acid) (thiol blocker), and thrombomodulin (anticoagulant) blocked S-protein-induced vWF, IL-6, and cit-H3. This suggests that following SARS-CoV-2 contact with the pulmonary endothelium or neutrophils and endothelial-neutrophil interactions, S-proteins increase adhesion molecules, induce endothelial injury, inflammation, NETosis and coagulopathy via the tissue factor pathway, mechanisms involving functional thiol groups, and/or the fibrinolysis system. Using rTFPI, effectors of the fibrinolysis system and/or thiol-based drugs could be viable therapeutic strategies against SARS-CoV-2-induced endothelial injury, inflammation, NETosis, and coagulopathy.
Keywords: DTNB; IL-6; P-selectin; SARS-CoV-2 spike proteins; TFPI; citrullinated histone H3; human lung endothelial cells; neutrophils; neutrophils extracellular traps; thrombomodulin; von willebrand factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- JHU Coronavirus Resource Center: COVID-19 in the USA. 2023. [(accessed on 24 April 2023)]. Available online: https://coronavirus.jhu.edu/
-
- CDC . United States COVID-19 Cases and Deaths by State. US Center for Disease Control and Prevention; Atlanta, GA, USA: 2023. [(accessed on 24 April 2023)]. Available online: https://www.cdc.gov/covid-data-tracker/#cases.
-
- WHO . Coronavirus Disease (COVID-19) Pandemic. World Health Organization; Geneva, Switzerland: 2023. [(accessed on 24 April 2023)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

