The Role of Specialized Pro-Resolving Lipid Mediators in Inflammation-Induced Carcinogenesis
- PMID: 37628804
- PMCID: PMC10454572
- DOI: 10.3390/ijms241612623
The Role of Specialized Pro-Resolving Lipid Mediators in Inflammation-Induced Carcinogenesis
Abstract
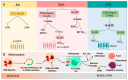
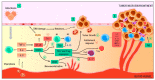
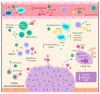
Cancer is a process involving cell mutation, increased proliferation, invasion, and metastasis. Over the years, this condition has represented one of the most concerning health problems worldwide due to its significant morbidity and mortality. At present, the incidence of cancer continues to grow exponentially. Thus, it is imperative to open new avenues in cancer research to understand the molecular changes driving DNA transformation, cell-to-cell interaction derangements, and immune system surveillance decay. In this regard, evidence supports the relationship between chronic inflammation and cancer. In light of this, a group of bioactive lipids derived from polyunsaturated fatty acids (PUFAs) may have a position as novel anti-inflammatory molecules known as the specialized pro-resolving mediators (SPMs), a group of pro-resolutive inflammation agents that could improve the anti-tumor immunity. These molecules have the potential role of chemopreventive and therapeutic agents for various cancer types, and their effects have been documented in the scientific literature. Thus, this review objective centers around understanding the effect of SPMs on carcinogenesis and their potential therapeutic effect.
Keywords: bioactive lipids; cancer; carcinogenesis; chronic inflammation; polyunsaturated fatty acids; specialized pro-resolving lipid mediators.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

