Osteoblast-Derived Matrix Vesicles Exhibit Exosomal Traits and a Unique Subset of microRNA: Their Caveolae-Dependent Endocytosis Results in Reduced Osteogenic Differentiation
- PMID: 37628952
- PMCID: PMC10454939
- DOI: 10.3390/ijms241612770
Osteoblast-Derived Matrix Vesicles Exhibit Exosomal Traits and a Unique Subset of microRNA: Their Caveolae-Dependent Endocytosis Results in Reduced Osteogenic Differentiation
Abstract
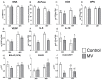
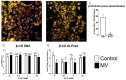
Matrix vesicles (MVs) are nano-sized extracellular vesicles that are anchored in the extracellular matrix (ECM). In addition to playing a role in biomineralization, osteoblast-derived MVs were recently suggested to have regulatory duties. The aims of this study were to establish the characteristics of osteoblast-derived MVs in the context of extracellular vesicles like exosomes, assess their role in modulating osteoblast differentiation, and examine their mechanism of uptake. MVs were isolated from the ECM of MG63 human osteoblast-like cell cultures and characterized via enzyme activity, transmission electron microscopy, nanoparticle tracking analysis, Western blot, and small RNA sequencing. Osteoblasts were treated with MVs from two different culture conditions (growth media [GM]; osteogenic media [OM]) to evaluate their effects on the differentiation and production of inflammatory markers and on macrophage polarization. MV endocytosis was assessed using a lipophilic, fluorescent dye and confocal microscopy with the role of caveolae determined using methyl-β-cyclodextrin. MVs exhibited a four-fold enrichment in alkaline phosphatase specific activity compared to plasma membranes; were 50-150 nm in diameter; possessed exosomal markers CD63, CD81, and CD9 and endosomal markers ALIX, TSG101, and HSP70; and were selectively enriched in microRNA linked to an anti-osteogenic effect and to M2 macrophage polarization. Treatment with GM or OM MVs decreased osteoblast differentiation. Osteoblasts endocytosed MVs using a mechanism that involves caveolae. These results support the hypothesis that osteoblasts produce MVs that participate in the regulation of osteogenesis.
Keywords: caveolin; differentiation; endocytosis; extracellular vesicles; matrix vesicles; microRNA; osteoblasts; osteogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Dean D.D., Schwartz Z., Bonewald L., Muniz O.E., Morales S., Gomez R., Brooks B.P., Qiao M., Howell D.S., Boyan B.D. Matrix vesicles produced by osteoblast-like cells in culture become significantly enriched in proteoglycan-degrading metalloproteinases after addition of β-Glycerophosphate and ascorbic acid. Calcif. Tissue Int. 1994;54:399–408. doi: 10.1007/BF00305527. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

