Beyond the Epidermal-Melanin-Unit: The Human Scalp Anagen Hair Bulb Is Home to Multiple Melanocyte Subpopulations of Variable Melanogenic Capacity
- PMID: 37628992
- PMCID: PMC10454394
- DOI: 10.3390/ijms241612809
Beyond the Epidermal-Melanin-Unit: The Human Scalp Anagen Hair Bulb Is Home to Multiple Melanocyte Subpopulations of Variable Melanogenic Capacity
Abstract
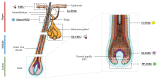
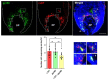
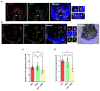
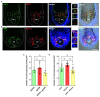
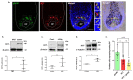
The visual appearance of humans is derived significantly from our skin and hair color. While melanin from epidermal melanocytes protects our skin from the damaging effects of ultraviolet radiation, the biological value of pigmentation in the hair follicle, particularly on the scalp, is less clear. In this study, we explore the heterogeneity of pigment cells in the human scalp anagen hair follicle bulb, a site conventionally viewed to be focused solely on pigment production for transfer to the hair shaft. Using c-KIT/CD117 microbeads, we isolated bulbar c-KIT-positive and c-KIT-negative melanocytes. While both subpopulations expressed MITF, only the c-KIT-positive fraction expressed SOX10. We further localized bulbar melanocyte subpopulations (expressing c-KIT, SOX10, MITF, and DCT) that exhibited distinct/variable expression of downstream differentiation-associated melanosome markers (e.g., gp100 and Melan-A). The localization of a second 'immature' SOX10 negative melanocyte population, which was c-KIT/MITF double-positive, was identified outside of the melanogenic zone in the most peripheral/proximal matrix. This study describes an approach to purifying human scalp anagen hair bulb melanocytes, allowing us to identify unexpected levels of melanocyte heterogeneity. The function of the more immature melanocytes in this part of the hair follicle remains to be elucidated. Could they be in-transit migratory cells ultimately destined to synthesize melanin, or could they contribute to the hair follicle in non-melanogenic ways?
Keywords: C-KIT; MITF; Melan-A; SOX10; bulbar melanocytes; follicular-melanin unit (FMU); hair follicle (HF); melanin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Slominski A., Paus R. Melanogenesis Is Coupled to Murine Anagen: Toward New Concepts for the Role of Melanocytes and the Regulation of Melanogenesis in Hair Growth. J. Investig. Dermatol. 1993;101:90S–97S. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

