Extracellular Cysteine Proteases of Key Intestinal Protozoan Pathogens-Factors Linked to Virulence and Pathogenicity
- PMID: 37629029
- PMCID: PMC10454693
- DOI: 10.3390/ijms241612850
Extracellular Cysteine Proteases of Key Intestinal Protozoan Pathogens-Factors Linked to Virulence and Pathogenicity
Abstract
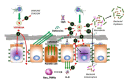
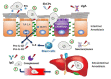
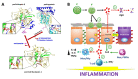
Intestinal diseases caused by protistan parasites of the genera Giardia (giardiasis), Entamoeba (amoebiasis), Cryptosporidium (cryptosporidiosis) and Blastocystis (blastocystosis) represent a major burden in human and animal populations worldwide due to the severity of diarrhea and/or inflammation in susceptible hosts. These pathogens interact with epithelial cells, promoting increased paracellular permeability and enterocyte cell death (mainly apoptosis), which precede physiological and immunological disorders. Some cell-surface-anchored and molecules secreted from these parasites function as virulence markers, of which peptide hydrolases, particularly cysteine proteases (CPs), are abundant and have versatile lytic activities. Upon secretion, CPs can affect host tissues and immune responses beyond the site of parasite colonization, thereby increasing the pathogens' virulence. The four intestinal protists considered here are known to secrete predominantly clan A (C1- and C2-type) CPs, some of which have been characterized. CPs of Giardia duodenalis (e.g., Giardipain-1) and Entamoeba histolytica (EhCPs 1-6 and EhCP112) degrade mucin and villin, cause damage to intercellular junction proteins, induce apoptosis in epithelial cells and degrade immunoglobulins, cytokines and defensins. In Cryptosporidium, five Cryptopains are encoded in its genome, but only Cryptopains 4 and 5 are likely secreted. In Blastocystis sp., a legumain-activated CP, called Blastopain-1, and legumain itself have been detected in the extracellular medium, and the former has similar adverse effects on epithelial integrity and enterocyte survival. Due to their different functions, these enzymes could represent novel drug targets. Indeed, some promising results with CP inhibitors, such as vinyl sulfones (K11777 and WRR605), the garlic derivative, allicin, and purified amoebic CPs have been obtained in experimental models, suggesting that these enzymes might be useful drug targets.
Keywords: cysteine proteases; extracellular proteases; intestinal protozoa; papain-like proteases; pathogenicity; protists; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Chalmers R.M. Chapter Sixteen—Cryptosporidium. In: Percival S.L., Yates M.V., Williams D.W., Chalmers R.M., Gray N.F., editors. Microbiology of Waterborne Diseases. 2nd ed. Academic Press; London, UK: 2014. pp. 287–326.
-
- Dogruman-Al F., Simsek Z., Boorom K., Ekici E., Sahin M., Tuncer C., Kustimur S., Altinbas A. Comparison of Methods for Detection of Blastocystis Infection in Routinely Submitted Stool Samples, and Also in IBS/IBD Patients in Ankara, Turkey. PLoS ONE. 2010;5:e15484. doi: 10.1371/journal.pone.0015484. - DOI - PMC - PubMed
-
- Argüello-García R., Carrero J.C., Ortega-Pierres G. Reference Module in Biomedical Sciences. Elsevier; Amsterdam, The Netherlands: 2020. Host Immune Responses Against Intestinal Unicellular Parasites and Their Role in Pathogenesis and Protection.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

