N-Acetyl-L-glutamate Kinase of Chlamydomonas reinhardtii: In Vivo Regulation by PII Protein and Beyond
- PMID: 37629055
- PMCID: PMC10454706
- DOI: 10.3390/ijms241612873
N-Acetyl-L-glutamate Kinase of Chlamydomonas reinhardtii: In Vivo Regulation by PII Protein and Beyond
Abstract
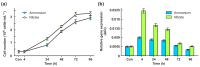
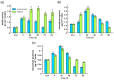
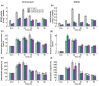
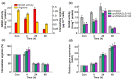
N-Acetyl-L-glutamate kinase (NAGK) catalyzes the rate-limiting step in the ornithine/arginine biosynthesis pathway in eukaryotic and bacterial oxygenic phototrophs. NAGK is the most highly conserved target of the PII signal transduction protein in Cyanobacteria and Archaeplastida (red algae and Chlorophyta). However, there is still much to be learned about how NAGK is regulated in vivo. The use of unicellular green alga Chlamydomonas reinhardtii as a model system has already been instrumental in identifying several key regulation mechanisms that control nitrogen (N) metabolism. With a combination of molecular-genetic and biochemical approaches, we show the existence of the complex CrNAGK control at the transcriptional level, which is dependent on N source and N availability. In growing cells, CrNAGK requires CrPII to properly sense the feedback inhibitor arginine. Moreover, we provide primary evidence that CrPII is only partly responsible for regulating CrNAGK activity to adapt to changing nutritional conditions. Collectively, our results suggest that in vivo CrNAGK is tuned at the transcriptional and post-translational levels, and CrPII and additional as yet unknown factor(s) are integral parts of this regulation.
Keywords: N-Acetyl-L-glutamate kinase; PII- signal transduction protein; arginine biosynthesis; green algae.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study, in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Klimaszewska K., Morency F., Jones-Overton C., Cooke J. Accumulation pattern and identification of seed storage proteins in zygotic embryos of Pinus strobus and in somatic embryos from different maturation treatments. Physiol. Plant. 2004;121:682–690. doi: 10.1111/j.1399-3054.2004.00370.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

