A Re-Purposing Strategy: Sub-Lethal Concentrations of an Eicosanoid Derived from the Omega-3-Polyunsaturated Fatty Acid Resolvin D1 Affect Dual Species Biofilms
- PMID: 37629056
- PMCID: PMC10454369
- DOI: 10.3390/ijms241612876
A Re-Purposing Strategy: Sub-Lethal Concentrations of an Eicosanoid Derived from the Omega-3-Polyunsaturated Fatty Acid Resolvin D1 Affect Dual Species Biofilms
Abstract
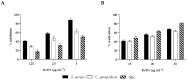
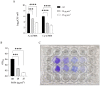
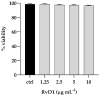
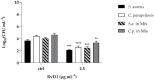
The fungal species Candida parapsilosis and the bacterial species Staphylococcus aureus may be responsible for hospital-acquired infections in patients undergoing invasive medical interventions or surgical procedures and often coinfect critically ill patients in complicating polymicrobial biofilms. The efficacy of the re-purposing therapy has recently been reported as an alternative to be used. PUFAs (polyunsaturated fatty acids) may be used alone or in combination with currently available traditional antimicrobials to prevent and manage various infections overcoming antimicrobial resistance. The objectives of the study were to evaluate the effects of Resolvin D1 (RvD1) as an antimicrobial on S. aureus and C. parapsilosis, as well as the activity against the mixed biofilm of the same two species. Microdilution assays and time-kill growth curves revealed bacterial and fungal inhibition at minimum concentration values between 5 and 10 μg mL-1. In single-species structures, an inhibition of 55% and 42% was reported for S. aureus and C. parapsilosis, respectively. Moreover, RvD1 demonstrated an eradication capacity of 60% and 80% for single- and mixed-species biofilms, respectively. In association with the inhibition activity, a downregulation of genes involved in biofilm formation as well as ROS accumulation was observed. Eradication capability was confirmed also on mature mixed biofilm grown on silicone platelets as shown by scanning electron microscopy (SEM). In conclusion, RvD1 was efficient against mono and polymicrobial biofilms in vitro, being a promising alternative for the treatment of mixed bacterial/fungal infections.
Keywords: Candida parapsilosis; Staphylococcus aureus; mixed biofilm; polyunsaturated fatty acids; resolvin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Antibiofilm efficacy of the gold compound auranofin on dual species biofilms of Staphylococcus aureus and Candida sp.J Appl Microbiol. 2020 Jan;128(1):88-101. doi: 10.1111/jam.14443. Epub 2019 Nov 19. J Appl Microbiol. 2020. PMID: 31509623
-
In vitro evaluation of biofilm formation by Candida parapsilosis and Enterobacter cloacae. Scanning electron microscopy and efficacy of antimicrobial combinations study.Diagn Microbiol Infect Dis. 2023 Sep;107(1):116003. doi: 10.1016/j.diagmicrobio.2023.116003. Epub 2023 Jun 8. Diagn Microbiol Infect Dis. 2023. PMID: 37423195
-
Eicosapentaenoic Acid and Docosahexaenoic Acid as an Antimicrobial Agent in Orthopedics-An In Vitro Study About the Race for Surface.Pathogens. 2025 Jan 10;14(1):57. doi: 10.3390/pathogens14010057. Pathogens. 2025. PMID: 39861018 Free PMC article.
-
Inhibition of Polymicrobial Biofilms of Candida albicans-Staphylococcus aureus/Streptococcus mutans by Fucoidan-Gold Nanoparticles.Mar Drugs. 2023 Feb 13;21(2):123. doi: 10.3390/md21020123. Mar Drugs. 2023. PMID: 36827164 Free PMC article.
-
Metabolic Adaptations During Staphylococcus aureus and Candida albicans Co-Infection.Front Immunol. 2021 Dec 8;12:797550. doi: 10.3389/fimmu.2021.797550. eCollection 2021. Front Immunol. 2021. PMID: 34956233 Free PMC article. Review.
Cited by
-
"Stop, Little Pot" as the Motto of Suppressive Management of Various Microbial Consortia.Microorganisms. 2024 Aug 12;12(8):1650. doi: 10.3390/microorganisms12081650. Microorganisms. 2024. PMID: 39203492 Free PMC article. Review.
-
Lipid mediators in neutrophil biology: inflammation, resolution and beyond.Curr Opin Hematol. 2024 Jul 1;31(4):175-192. doi: 10.1097/MOH.0000000000000822. Epub 2024 May 7. Curr Opin Hematol. 2024. PMID: 38727155 Free PMC article. Review.
-
Antibacterial and Antibiofilm Efficacy of Phenyllactic Acid Against Foodborne Pathogens Salmonella enterica Serotype Derby and Escherichia coli O26.Molecules. 2025 Apr 13;30(8):1738. doi: 10.3390/molecules30081738. Molecules. 2025. PMID: 40333660 Free PMC article.
-
Anti-bacterial and anti-biofilm activities of arachidonic acid against the cariogenic bacterium Streptococcus mutans.Front Microbiol. 2024 Feb 26;15:1333274. doi: 10.3389/fmicb.2024.1333274. eCollection 2024. Front Microbiol. 2024. PMID: 38596377 Free PMC article.
References
-
- Cangui-Panchi S.P., Ñacato-Toapanta A.L., Enríquez-Martínez L.J., Reyes J., Garzon-Chavez D., Machado A. Biofilm-forming microorganisms causing hospital-acquired infections from intravenous catheter: A systematic review. Curr. Res. Microb. Sci. 2022;3:100175. doi: 10.1016/j.crmicr.2022.100175. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

