Regulation of Smooth Muscle Cell Proliferation by Mitochondrial Ca2+ in Type 2 Diabetes
- PMID: 37629079
- PMCID: PMC10454141
- DOI: 10.3390/ijms241612897
Regulation of Smooth Muscle Cell Proliferation by Mitochondrial Ca2+ in Type 2 Diabetes
Abstract
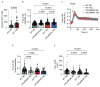
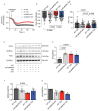
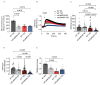
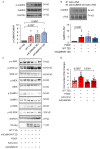
Type 2 diabetes (T2D) is associated with increased risk of atherosclerotic vascular disease due to excessive vascular smooth muscle cell (VSMC) proliferation. Here, we investigated the role of mitochondrial dysfunction and Ca2+ levels in VSMC proliferation in T2D. VSMCs were isolated from normoglycemic and T2D-like mice induced by diet. The effects of mitochondrial Ca2+ uptake were studied using mice with selectively inhibited mitochondrial Ca2+/calmodulin-dependent kinase II (mtCaMKII) in VSMCs. Mitochondrial transition pore (mPTP) was blocked using ER-000444793. VSMCs from T2D compared to normoglycemic mice exhibited increased proliferation and baseline cytosolic Ca2+ levels ([Ca2+]cyto). T2D cells displayed lower endoplasmic reticulum Ca2+ levels, reduced mitochondrial Ca2+ entry, and increased Ca2+ leakage through the mPTP. Mitochondrial and cytosolic Ca2+ transients were diminished in T2D cells upon platelet-derived growth factor (PDGF) administration. Inhibiting mitochondrial Ca2+ uptake or the mPTP reduced VSMC proliferation in T2D, but had contrasting effects on [Ca2+]cyto. In T2D VSMCs, enhanced activation of Erk1/2 and its upstream regulators was observed, driven by elevated [Ca2+]cyto. Inhibiting mtCaMKII worsened the Ca2+ imbalance by blocking mitochondrial Ca2+ entry, leading to further increases in [Ca2+]cyto and Erk1/2 hyperactivation. Under these conditions, PDGF had no effect on VSMC proliferation. Inhibiting Ca2+-dependent signaling in the cytosol reduced excessive Erk1/2 activation and VSMC proliferation. Our findings suggest that altered Ca2+ handling drives enhanced VSMC proliferation in T2D, with mitochondrial dysfunction contributing to this process.
Keywords: calcium; proliferation; type 2 diabetes; vascular smooth muscle cells.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


Update of
-
The mitochondrial regulation of smooth muscle cell proliferation in type 2 diabetes.bioRxiv [Preprint]. 2023 Feb 16:2023.02.15.528765. doi: 10.1101/2023.02.15.528765. bioRxiv. 2023. Update in: Int J Mol Sci. 2023 Aug 17;24(16):12897. doi: 10.3390/ijms241612897. PMID: 36824758 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

