Neurovascular Cell Death and Therapeutic Strategies for Diabetic Retinopathy
- PMID: 37629100
- PMCID: PMC10454228
- DOI: 10.3390/ijms241612919
Neurovascular Cell Death and Therapeutic Strategies for Diabetic Retinopathy
Abstract
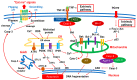
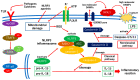
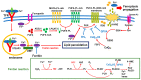
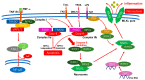
Diabetic retinopathy (DR) is a major complication of diabetes and a leading cause of blindness worldwide. DR was recently defined as a neurovascular disease associated with tissue-specific neurovascular impairment of the retina in patients with diabetes. Neurovascular cell death is the main cause of neurovascular impairment in DR. Thus, neurovascular cell protection is a potential therapy for preventing the progression of DR. Growing evidence indicates that a variety of cell death pathways, such as apoptosis, necroptosis, ferroptosis, and pyroptosis, are associated with neurovascular cell death in DR. These forms of regulated cell death may serve as therapeutic targets for ameliorating the pathogenesis of DR. This review focuses on these cell death mechanisms and describes potential therapies for the treatment of DR that protect against neurovascular cell death.
Keywords: apoptosis; diabetic retinopathy; ferroptosis; necroptosis; neuroprotection; neurovascular cell death; neurovascular unit; pyroptosis; vasoprotection.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the study design; collection, analyses, or interpretation of data; writing of the manuscript; or decision to publish the results.
Figures
Similar articles
-
Neurovascular Impairment and Therapeutic Strategies in Diabetic Retinopathy.Int J Environ Res Public Health. 2021 Dec 31;19(1):439. doi: 10.3390/ijerph19010439. Int J Environ Res Public Health. 2021. PMID: 35010703 Free PMC article. Review.
-
Pyroptosis in the Retinal Neurovascular Unit: New Insights Into Diabetic Retinopathy.Front Immunol. 2021 Oct 19;12:763092. doi: 10.3389/fimmu.2021.763092. eCollection 2021. Front Immunol. 2021. PMID: 34737754 Free PMC article. Review.
-
Targeting Novel Regulated Cell Death: Pyroptosis, Necroptosis, and Ferroptosis in Diabetic Retinopathy.Front Cell Dev Biol. 2022 Jun 23;10:932886. doi: 10.3389/fcell.2022.932886. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35813208 Free PMC article. Review.
-
The Pathogenesis and Therapeutic Approaches of Diabetic Neuropathy in the Retina.Int J Mol Sci. 2021 Aug 22;22(16):9050. doi: 10.3390/ijms22169050. Int J Mol Sci. 2021. PMID: 34445756 Free PMC article. Review.
-
Advanced Glycation End-Products and Diabetic Neuropathy of the Retina.Int J Mol Sci. 2023 Feb 2;24(3):2927. doi: 10.3390/ijms24032927. Int J Mol Sci. 2023. PMID: 36769249 Free PMC article. Review.
Cited by
-
Translational Research and Therapies for Neuroprotection and Regeneration of the Optic Nerve and Retina: A Narrative Review.Int J Mol Sci. 2024 Sep 29;25(19):10485. doi: 10.3390/ijms251910485. Int J Mol Sci. 2024. PMID: 39408817 Free PMC article. Review.
-
Resveratrol Protects Müller Cells Against Ferroptosis in the Early Stage of Diabetic Retinopathy by Regulating the Nrf2/GPx4/PTGS2 Pathway.Mol Neurobiol. 2025 Mar;62(3):3412-3427. doi: 10.1007/s12035-024-04496-8. Epub 2024 Sep 18. Mol Neurobiol. 2025. PMID: 39292340
-
Ghrelin alleviates high glucose-induced retinal microvascular endothelial cell injury by activating Nrf2/HO-1 pathway to inhibit ferroptosis.Int J Ophthalmol. 2025 Jun 18;18(6):978-985. doi: 10.18240/ijo.2025.06.02. eCollection 2025. Int J Ophthalmol. 2025. PMID: 40534808 Free PMC article.
-
Rejuvenation of diabetic macular edema with senolytic therapy.Nat Med. 2024 Feb;30(2):346-347. doi: 10.1038/s41591-024-02804-2. Nat Med. 2024. PMID: 38332040 No abstract available.
-
Microvascular destabilization and intricated network of the cytokines in diabetic retinopathy: from the perspective of cellular and molecular components.Cell Biosci. 2024 Jun 27;14(1):85. doi: 10.1186/s13578-024-01269-7. Cell Biosci. 2024. PMID: 38937783 Free PMC article. Review.
References
-
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C., Basit A., Chan J.C.N., Mbanya J.C., et al. IDF Diabetes Atlas: Global, regional, and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. - DOI - PMC - PubMed
-
- Teo Z.L., Tham Y.C., Yu M., Chee M.L., Rim T.H., Cheung N., Bikbov M.M., Wang Y.X., Tang Y., Lu Y., et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580–1591. doi: 10.1016/j.ophtha.2021.04.027. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

