Heterodimerization of Chemoreceptors TAS1R3 and mGlu2 in Human Blood Leukocytes
- PMID: 37629122
- PMCID: PMC10454557
- DOI: 10.3390/ijms241612942
Heterodimerization of Chemoreceptors TAS1R3 and mGlu2 in Human Blood Leukocytes
Abstract
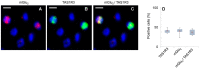
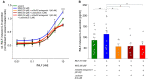
The expression of canonical chemosensory receptors of the tongue, such as the heteromeric sweet taste (TAS1R2/TAS1R3) and umami taste (TAS1R1/TAS1R3) receptors, has been demonstrated in many extra-oral cells and tissues. Gene expression studies have revealed transcripts for all TAS1 and metabotropic glutamate (mGlu) receptors in different types of immune cells, where they are involved, for example, in the chemotaxis of human neutrophils and the protection of T cells from activation-induced cell death. Like other class-C G protein-coupling receptors (GPCRs), TAS1Rs and mGlu receptors form heteromers within their families. Since mGlu receptors and TAS1R1/TAS1R3 share the same ligand, monosodium glutamate (MSG), we hypothesized their hitherto unknown heteromerization across receptor families in leukocytes. Here we show, by means of immunocytochemistry and co-IP/Western analysis, that across class-C GPCR families, mGlu2 and TAS1R3 co-localize and heterodimerize in blood leukocytes. Expressing the recombinant receptors in HEK-293 cells, we validated their heterodimerization by bioluminescence resonance energy transfer. We demonstrate MSG-induced, mGlu2/TAS1R3 heteromer-dependent gain-of-function and pertussis toxin-sensitive signaling in luminescence assays. Notably, we show that mGlu2/TAS1R3 is necessary and sufficient for MSG-induced facilitation of N-formyl-methionyl-leucyl-phenylalanine-stimulated IL-8 secretion in neutrophils, using receptor-specific antagonists. In summary, our results demonstrate mGlu2/TAS1R3 heterodimerization in leukocytes, suggesting cellular function-tailored chemoreceptor combinations to modulate cellular immune responses.
Keywords: BRET; ELISA; calcium fluorescence flow cytometry; chemosensory; fMLF; immunocytochemistry; taste receptors; transcript regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Nonsynonymous single nucleotide polymorphisms in human tas1r1, tas1r3, and mGluR1 and individual taste sensitivity to glutamate.Am J Clin Nutr. 2009 Sep;90(3):789S-799S. doi: 10.3945/ajcn.2009.27462P. Epub 2009 Jul 1. Am J Clin Nutr. 2009. PMID: 19571223
-
Tas1R1-Tas1R3 taste receptor variants in human fungiform papillae.Neurosci Lett. 2009 Feb 27;451(3):217-21. doi: 10.1016/j.neulet.2008.12.060. Epub 2009 Jan 6. Neurosci Lett. 2009. PMID: 19146926
-
Inosine-5'-monophosphate interacts with the TAS1R3 subunit to enhance sweet taste detection.Food Chem (Oxf). 2025 Feb 11;10:100246. doi: 10.1016/j.fochms.2025.100246. eCollection 2025 Jun. Food Chem (Oxf). 2025. PMID: 40034539 Free PMC article.
-
Variation in umami perception and in candidate genes for the umami receptor in mice and humans.Am J Clin Nutr. 2009 Sep;90(3):764S-769S. doi: 10.3945/ajcn.2009.27462M. Epub 2009 Jul 22. Am J Clin Nutr. 2009. PMID: 19625681 Free PMC article. Review.
-
Structure, function, and signaling of taste G-protein-coupled receptors.Curr Pharm Biotechnol. 2014;15(10):951-61. doi: 10.2174/1389201015666140922105911. Curr Pharm Biotechnol. 2014. PMID: 25248559 Review.
References
-
- Borroto-Escuela D.O., Brito I., Romero-Fernandez W., Di Palma M., Oflijan J., Skieterska K., Duchou J., Van Craenenbroeck K., Suarez-Boomgaard D., Rivera A., et al. The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub components. Int. J. Mol. Sci. 2014;15:8570–8590. doi: 10.3390/ijms15058570. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

