Calcium Signaling during Cortical Apical Dendrite Initiation: A Role for Cajal-Retzius Neurons
- PMID: 37629145
- PMCID: PMC10455361
- DOI: 10.3390/ijms241612965
Calcium Signaling during Cortical Apical Dendrite Initiation: A Role for Cajal-Retzius Neurons
Abstract
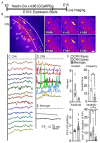
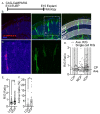
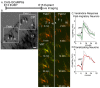
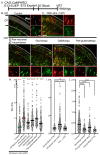
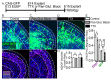
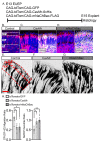
The apical dendrite of a cortical projection neuron (CPN) is generated from the leading process of the migrating neuron as the neuron completes migration. This transformation occurs in the cortical marginal zone (MZ), a layer that contains the Cajal-Retzius neurons and their axonal projections. Cajal-Retzius neurons (CRNs) are well known for their critical role in secreting Reelin, a glycoprotein that controls dendritogenesis and cell positioning in many regions of the developing brain. In this study, we examine the possibility that CRNs in the MZ may provide additional signals to arriving CPNs, that may promote the maturation of CPNs and thus shape the development of the cortex. We use whole embryonic hemisphere explants and multiphoton microscopy to confirm that CRNs display intracellular calcium transients of <1-min duration and high amplitude during early corticogenesis. In contrast, developing CPNs do not show high-amplitude calcium transients, but instead show a steady increase in intracellular calcium that begins at the time of dendritic initiation, when the leading process of the migrating CPN is encountering the MZ. The possible existence of CRN to CPN communication was revealed by the application of veratridine, a sodium channel activator, which has been shown to preferentially stimulate more mature cells in the MZ at an early developmental time. Surprisingly, veratridine application also triggers large calcium transients in CPNs, which can be partially blocked by a cocktail of antagonists that block glutamate and glycine receptor activation. These findings outline a model in which CRN spontaneous activity triggers the release of glutamate and glycine, neurotransmitters that can trigger intracellular calcium elevations in CPNs. These elevations begin as CPNs initiate dendritogenesis and continue as waves in the post-migratory cells. Moreover, we show that the pharmacological blockade of glutamatergic signaling disrupts migration, while forced expression of a bacterial voltage-gated calcium channel (CavMr) in the migrating neurons promotes dendritic growth and migration arrest. The identification of CRN to CPN signaling during early development provides insight into the observation that many autism-linked genes encode synaptic proteins that, paradoxically, are expressed in the developing cortex well before the appearance of synapses and the establishment of functional circuits.
Keywords: Cajal-Retzius neurons; cortical projection neurons; dendritogenesis; glutamate; glycine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Reelin Prevents Apical Neurite Retraction during Terminal Translocation and Dendrite Initiation.J Neurosci. 2015 Jul 29;35(30):10659-74. doi: 10.1523/JNEUROSCI.1629-15.2015. J Neurosci. 2015. PMID: 26224852 Free PMC article.
-
Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin.Brain Res Dev Brain Res. 2003 Mar 14;141(1-2):39-53. doi: 10.1016/s0165-3806(02)00641-7. Brain Res Dev Brain Res. 2003. PMID: 12644247
-
Reelin Signaling Inactivates Cofilin to Stabilize the Cytoskeleton of Migrating Cortical Neurons.Front Cell Neurosci. 2017 May 23;11:148. doi: 10.3389/fncel.2017.00148. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28588454 Free PMC article. Review.
-
Glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the murine cortex as identified with calretinin antibody.Cereb Cortex. 1995 Jan-Feb;5(1):13-21. doi: 10.1093/cercor/5.1.13. Cereb Cortex. 1995. PMID: 7719127
-
Cajal, Retzius, and Cajal-Retzius cells.Front Neuroanat. 2014 Jun 17;8:48. doi: 10.3389/fnana.2014.00048. eCollection 2014. Front Neuroanat. 2014. PMID: 24987337 Free PMC article. Review.
Cited by
-
Reelin differentially shapes dendrite morphology of medial entorhinal cortical ocean and island cells.Development. 2024 Jul 1;151(13):dev202449. doi: 10.1242/dev.202449. Epub 2024 Jun 27. Development. 2024. PMID: 38856043 Free PMC article.
-
Interplay of Vitamin D, Unfolded Protein Response, and Iron Metabolism in Neuroblastoma Cells: A Therapeutic Approach in Neurodegenerative Conditions.Int J Mol Sci. 2023 Nov 28;24(23):16883. doi: 10.3390/ijms242316883. Int J Mol Sci. 2023. PMID: 38069206 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

