Ubiquitination Is a Novel Post-Translational Modification of VMP1 in Autophagy of Human Tumor Cells
- PMID: 37629161
- PMCID: PMC10455450
- DOI: 10.3390/ijms241612981
Ubiquitination Is a Novel Post-Translational Modification of VMP1 in Autophagy of Human Tumor Cells
Abstract
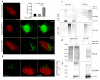
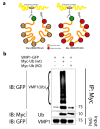
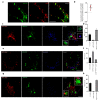
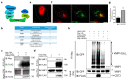
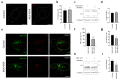
Autophagy is a tightly regulated catabolic process involved in the degradation and recycling of proteins and organelles. Ubiquitination plays an important role in the regulation of autophagy. Vacuole Membrane Protein 1 (VMP1) is an essential autophagy protein. The expression of VMP1 in pancreatic cancer stem cells carrying the activated Kirsten rat sarcoma viral oncogene homolog (KRAS) triggers autophagy and enables therapy resistance. Using biochemical and cellular approaches, we identified ubiquitination as a post-translational modification of VMP1 from the initial steps in autophagosome biogenesis. VMP1 remains ubiquitinated as part of the autophagosome membrane throughout autophagic flux until autolysosome formation. However, VMP1 is not degraded by autophagy, nor by the ubiquitin-proteasomal system. Mass spectrometry and immunoprecipitation showed that the cell division cycle protein cdt2 (Cdt2), the substrate recognition subunit of the E3 ligase complex associated with cancer, cullin-RING ubiquitin ligase complex 4 (CRL4), is a novel interactor of VMP1 and is involved in VMP1 ubiquitination. VMP1 ubiquitination decreases under the CRL inhibitor MLN4924 and increases with Cdt2 overexpression. Moreover, VMP1 recruitment and autophagosome formation is significantly affected by CRL inhibition. Our results indicate that ubiquitination is a novel post-translational modification of VMP1 during autophagy in human tumor cells. VMP1 ubiquitination may be of clinical relevance in tumor-cell-therapy resistance.
Keywords: DFCP1; VMP1; autophagosome; tumor cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

