Interleukin 6 (IL-6) Regulates GABAA Receptors in the Dorsomedial Hypothalamus Nucleus (DMH) through Activation of the JAK/STAT Pathway to Affect Heart Rate Variability in Stressed Rats
- PMID: 37629166
- PMCID: PMC10455568
- DOI: 10.3390/ijms241612985
Interleukin 6 (IL-6) Regulates GABAA Receptors in the Dorsomedial Hypothalamus Nucleus (DMH) through Activation of the JAK/STAT Pathway to Affect Heart Rate Variability in Stressed Rats
Abstract
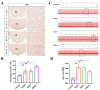
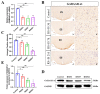
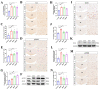
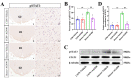
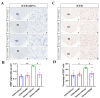
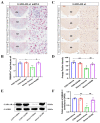
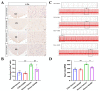
The dorsomedial hypothalamus nucleus (DMH) is an important component of the autonomic nervous system and plays a critical role in regulating the sympathetic outputs of the heart. Stress alters the neuronal activity of the DMH, affecting sympathetic outputs and triggering heart rate variability. However, the specific molecular mechanisms behind stress leading to abnormal DMH neuronal activity have still not been fully elucidated. Therefore, in the present study, we successfully constructed a stressed rat model and used it to investigate the potential molecular mechanisms by which IL-6 regulates GABAA receptors in the DMH through activation of the JAK/STAT pathway and thus affects heart rate variability in rats. By detecting the c-Fos expression of neurons in the DMH and electrocardiogram (ECG) changes in rats, we clarified the relationship between abnormal DMH neuronal activity and heart rate variability in stressed rats. Then, using ELISA, immunohistochemical staining, Western blotting, RT-qPCR, and RNAscope, we further explored the correlation between the IL-6/JAK/STAT signaling pathway and GABAA receptors. The data showed that an increase in IL-6 induced by stress inhibited GABAA receptors in DMH neurons by activating the JAK/STAT signaling pathway, while specific inhibition of the JAK/STAT signaling pathway using AG490 obviously reduced DMH neuronal activity and improved heart rate variability in rats. These findings suggest that IL-6 regulates the expression of GABAA receptors via the activation of the JAK/STAT pathway in the DMH, which may be an important cause of heart rate variability in stressed rats.
Keywords: GABAA receptor; JAK/STAT; dorsomedial hypothalamus nucleus; heart rate; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Liujunzi Decoction ameliorated cisplatin-induced anorexia by inhibiting the JAK-STAT signaling pathway and coordinating anorexigenic and orexigenic neuropeptides in rats.J Ethnopharmacol. 2022 Mar 1;285:114840. doi: 10.1016/j.jep.2021.114840. Epub 2021 Nov 17. J Ethnopharmacol. 2022. PMID: 34800646
-
JAK/STAT pathway regulation of GABAA receptor expression after differing severities of experimental TBI.Exp Neurol. 2015 Sep;271:445-56. doi: 10.1016/j.expneurol.2015.07.001. Epub 2015 Jul 11. Exp Neurol. 2015. PMID: 26172316 Free PMC article.
-
IL-11 ameliorates oxidative stress damage in neurons after spinal cord injury by activating the JAK/STAT signaling pathway.Int Immunopharmacol. 2024 Jan 25;127:111367. doi: 10.1016/j.intimp.2023.111367. Epub 2023 Dec 30. Int Immunopharmacol. 2024. PMID: 38160564
-
Stress-induced cardiac stimulation and fever: common hypothalamic origins and brainstem mechanisms.Auton Neurosci. 2006 Jun 30;126-127:106-19. doi: 10.1016/j.autneu.2006.02.010. Epub 2006 Apr 3. Auton Neurosci. 2006. PMID: 16580890 Review.
-
The involvement of JAK/STAT signaling pathway in the treatment of Parkinson's disease.J Neuroimmunol. 2021 Dec 15;361:577758. doi: 10.1016/j.jneuroim.2021.577758. Epub 2021 Oct 28. J Neuroimmunol. 2021. PMID: 34739911 Review.
Cited by
-
Exploring anesthetic-induced gene expression changes and immune cell dynamics in atrial tissue post-coronary artery bypass graft surgery.Open Med (Wars). 2024 Aug 13;19(1):20241014. doi: 10.1515/med-2024-1014. eCollection 2024. Open Med (Wars). 2024. PMID: 39156756 Free PMC article.
-
The TF/Nrf2/GSTP1 pathway is involved in stress-induced hepatocellular injury through ferroptosis.J Cell Mol Med. 2024 Jun;28(12):e18494. doi: 10.1111/jcmm.18494. J Cell Mol Med. 2024. PMID: 38890797 Free PMC article.
-
Pathological changes in the spleen of mice subjected to different time courses of restraint stress.Sci Rep. 2024 Jun 12;14(1):13543. doi: 10.1038/s41598-024-64475-w. Sci Rep. 2024. PMID: 38866996 Free PMC article.
-
A diagnostic index for predicting heart rate variability decline and prognostic value in newly diagnosed non-small cell lung cancer patients.Front Oncol. 2024 Dec 4;14:1463805. doi: 10.3389/fonc.2024.1463805. eCollection 2024. Front Oncol. 2024. PMID: 39697224 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

