Investigational Drugs for the Treatment of Postherpetic Neuralgia: Systematic Review of Randomized Controlled Trials
- PMID: 37629168
- PMCID: PMC10455720
- DOI: 10.3390/ijms241612987
Investigational Drugs for the Treatment of Postherpetic Neuralgia: Systematic Review of Randomized Controlled Trials
Abstract
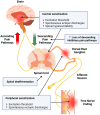
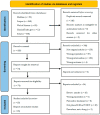
The pharmacological treatment of postherpetic neuralgia (PHN) is unsatisfactory, and there is a clinical need for new approaches. Several drugs under advanced clinical development are addressed in this review. A systematic literature search was conducted in three electronic databases (Medline, Web of Science, Scopus) and in the ClinicalTrials.gov register from 1 January 2016 to 1 June 2023 to identify Phase II, III and IV clinical trials evaluating drugs for the treatment of PHN. A total of 18 clinical trials were selected evaluating 15 molecules with pharmacological actions on nine different molecular targets: Angiotensin Type 2 Receptor (AT2R) antagonism (olodanrigan), Voltage-Gated Calcium Channel (VGCC) α2δ subunit inhibition (crisugabalin, mirogabalin and pregabalin), Voltage-Gated Sodium Channel (VGSC) blockade (funapide and lidocaine), Cyclooxygenase-1 (COX-1) inhibition (TRK-700), Adaptor-Associated Kinase 1 (AAK1) inhibition (LX9211), Lanthionine Synthetase C-Like Protein (LANCL) activation (LAT8881), N-Methyl-D-Aspartate (NMDA) receptor antagonism (esketamine), mu opioid receptor agonism (tramadol, oxycodone and hydromorphone) and Nerve Growth Factor (NGF) inhibition (fulranumab). In brief, there are several drugs in advanced clinical development for treating PHN with some of them reporting promising results. AT2R antagonism, AAK1 inhibition, LANCL activation and NGF inhibition are considered first-in-class analgesics. Hopefully, these trials will result in a better clinical management of PHN.
Keywords: AAK1; AT2R; COX; LANCL; NMDA; analgesic; first in class; nerve growth factor; neuropathic pain; opioid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Modeling an evaluation of the efficacy of the novel neuroanalgesic drug mirogabalin for diabetic peripheral neuropathic pain and postherpetic neuralgia therapy.Eur J Pharm Sci. 2024 Jun 1;197:106777. doi: 10.1016/j.ejps.2024.106777. Epub 2024 Apr 20. Eur J Pharm Sci. 2024. PMID: 38649099
-
Pregabalin treatment for peripheral neuropathic pain: a review of safety data from randomized controlled trials conducted in Japan and in the west.Drug Saf. 2012 Oct 1;35(10):793-806. doi: 10.2165/11632660-000000000-00000. Drug Saf. 2012. PMID: 22967187 Review.
-
Efficacy and safety of 5% lidocaine (lignocaine) medicated plaster in comparison with pregabalin in patients with postherpetic neuralgia and diabetic polyneuropathy: interim analysis from an open-label, two-stage adaptive, randomized, controlled trial.Clin Drug Investig. 2009;29(4):231-41. doi: 10.2165/00044011-200929040-00002. Clin Drug Investig. 2009. PMID: 19301937 Clinical Trial.
-
Mirogabalin-A Novel Selective Ligand for the α2δ Calcium Channel Subunit.Pharmaceuticals (Basel). 2021 Jan 31;14(2):112. doi: 10.3390/ph14020112. Pharmaceuticals (Basel). 2021. PMID: 33572689 Free PMC article. Review.
-
A retrospective evaluation of the use of gabapentin and pregabalin in patients with postherpetic neuralgia in usual-care settings.Clin Ther. 2007 Aug;29(8):1655-70. doi: 10.1016/j.clinthera.2007.08.019. Clin Ther. 2007. PMID: 17919547
Cited by
-
Different Acupuncture Therapies for Postherpetic Neuralgia: An Overview of Systematic Reviews and Meta-analysis.Chin J Integr Med. 2025 Jan;31(1):55-67. doi: 10.1007/s11655-023-3613-4. Epub 2024 Jan 12. Chin J Integr Med. 2025. PMID: 38212497
-
Efficacy and Safety of Cold Atmospheric Plasma-Assisted Therapy for Herpes Zoster: A Randomized, Parallel, Positive-Controlled, Non-inferiority Multicenter Clinical Trial.Dermatol Ther (Heidelb). 2025 Sep;15(9):2391-2408. doi: 10.1007/s13555-025-01467-2. Epub 2025 Jul 2. Dermatol Ther (Heidelb). 2025. PMID: 40601233 Free PMC article.
-
Endophilin A1 and synaptojanin 1-dependent endocytosis of synaptic vesicles in nociceptive spinal circuits maintains postoperative and cancer pain.Pain. 2025 May 27:10.1097/j.pain.0000000000003657. doi: 10.1097/j.pain.0000000000003657. Online ahead of print. Pain. 2025. PMID: 40488273
-
Ultrasound-guided thoracic paravertebral injection of dexamethasone palmitate combined with ropivacaine for the treatment of thoracic herpes zoster-related pain: protocol for a prospective, randomized controlled, single-center study.Front Pharmacol. 2025 Jan 7;15:1470772. doi: 10.3389/fphar.2024.1470772. eCollection 2024. Front Pharmacol. 2025. PMID: 39872051 Free PMC article.
-
Effects of Medical Ozone Injection via Intervertebral Foramen Epidural Space on Postherpetic Neuralgia: Protocol for a Randomized Controlled and Double-Blind Clinical Trial.JMIR Res Protoc. 2025 Jul 15;14:e68847. doi: 10.2196/68847. JMIR Res Protoc. 2025. PMID: 40663769 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials